Team:Edinburgh/Human Practices/Waste Treatment/Existing technology
From 2013.igem.org
Contents |
Waste water treatment
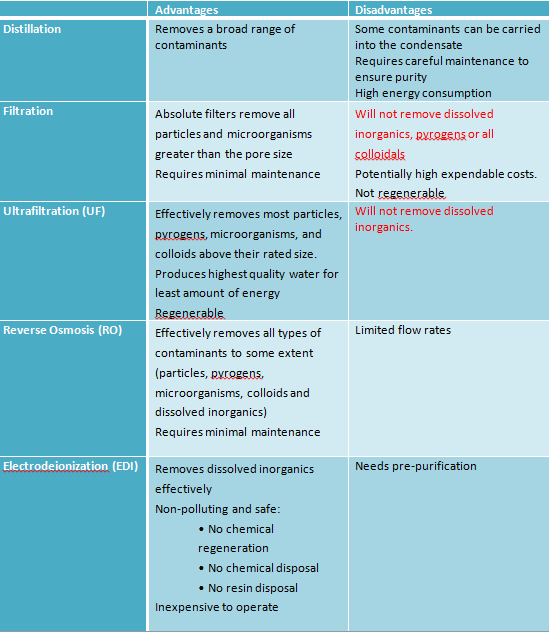
Distillation
Distillation is probably the oldest method for water purification. Water vapour rising from the boiling pot is condensed, collected and store in the condenser, leaving most contaminants in the liquid phase vessel. Organic contaminants with boiling point lower than that of water (e.g. alcohol) cannot be removed by this process. Also, energy consumption of distillation is high.
Filtration
Contaminants in water can be divided into suspended solids and dissolved solids. Filtration, as a water purification technique, can remove suspended solids completely as long as particle size and gravity is significant. During filtration, water passes through a porous membrane (filter, pore size depends on the filtration requirement) and the suspended solids will be retained and accumulated on the surface of the filter.
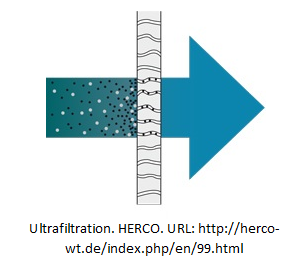
Ultrafiltration (UF)
Similar to filtration, ultrafiltration purifies water by using membrane to retain water contaminants. The fine filter (pore size 0.01~0.1µm) acts as a molecular sieve, effectively remove insoluble solids, colloids and microorganisms. The purification process requires pressures (increases gradually until backwash) supply to drive the feed through the membrane. However, ultrafiltration is not able to remove solvents (e.g. alcohol, ethanol) or dissolved inorganics.
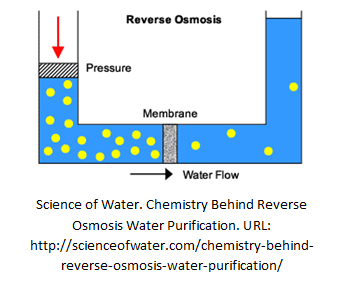
Reverse Osmosis (RO)
Osmosis happens when a saline solution and water are separated by a semi-permeable membrane. The water moves through the membrane and dilutes the saline solution until the concentration equivalence is achieved on both side.
Osmosis is a reversible process. If significant pressure (similar to UF, increases gradually) is applied on the side where saline solution is kept, pure water and a highly concentrated solution can be produced. With a much finer membrane than ultrafiltration, reverse osmosis can effectively remove 90 to 99% of all contaminants.
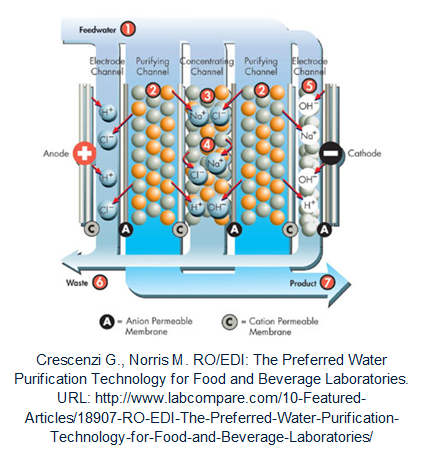
Eletrodeionization (EDI, Elix® )
Eletrodeionization is a process for continuous demineralization of pre-purified water. An EDI module consists of several chambers that are separated by ion-selective membranes (allow only anion or cation to go through). The chambers are filled with ion exchange resin and placed between two DC voltage electrodes. The ions contained in the EDI feed water are driven to the corresponding electrode by the voltage applied (Cations attracted by the cathode and anions by the anode). Ion-selective membranes are positioned inversely so that all ions flow into the channels between the chambers and then are collected and carried out of the module by the concentrate flow (at the middle in the figure below).
Particular advantages of this purification method are the process is uninterrupted and have no need for chemicals. Moreover, the EDI concentrate does not require neutralization; it can be recirculated within the unit or used for other purposes.
References
RSD®. Purification technologies. URL: http://www.ro-di.com/en/chjs.asp
HERCO. treatment. URL: http://herco-wt.de/index.php/en/methods.html
Crescenzi G., Norris M. RO/EDI: The Preferred Water Purification Technology for Food and Beverage Laboratories. URL: http://www.labcompare.com/10-Featured-Articles/18907-RO-EDI-The-Preferred-Water-Purification-Technology-for-Food-and-Beverage-Laboratories/

| 
| | | | 
|
| This iGEM team has been funded by the MSD Scottish Life Sciences Fund. The opinions expressed by this iGEM team are those of the team members and do not necessarily represent those of MSD | |||||
 "
"