Team:UC-Santa Cruz/Project/Biofilm
From 2013.igem.org
Biofilm Membrane
Formation of a continuous dense monolayer biofilm is at the heart of our biological desalination system. The biofilm separates two chambers, and mediates the unidirectional transport of ions from one chamber to the other by means of the recombinant pumps and channels engineered to localize to the two sides of the biofilm. In principle, a biofilm can selectively transport any molecule for which a set of inward and outward active transporters are available.
The goal of the biofilm team was to grow high-density monolayer biofilms of Caulobacter crescentus. This module would then be combined with the pump/polarization modules to produce a “bio-active biofilm” that can be tested for efficient ion transport by appropriate assays.
Caulobacter crescentus is a gram negative aquatic bacterium whose unusual life cycle has been extensively studied. It divides asymmetrically to produce a replicating ‘stalked’ cell that is normally attached to a substratum and an immature ‘swarmer’ cell that is motile. As the stalked cell prepares for cell division, it becomes polarized and expresses different sets of proteins at the two opposite poles of the cell; this ensures that the flagellum, for instance, is only formed at the distal pole of the cell that will give rise to the swarmer cell, and not at the proximal pole where the stalk is located.
Caulobacter crescentus, naturally growing in ponds and streams, can be grown in complex media such as PYE, or in defined media such as M2. It is commonly grown at 33 degrees Celsius with sufficient aeration. The wild-type strain CB15 is resistant to the antibiotic ampicillin, which can be added to the media to help prevent contamination.
We started with the simple task of growing the wild-type Caulobacter strain CB15 in complex media and characterizing its general growth features. At 33 degrees Celsius and with vigorous shaking, the optical density of the culture at 550 nm peaks at ~ 1.8 units by ~48 h, after which it decreases probably due to cell death.
We then grew Caulobacter biofilms. We cultured the cells on different surfaces (glass or plastic coverslips, or filter paper) under a number of conditions used previously to obtain monolayer biofilms, in order to find the conditions that result in the best biofilm morphology in our hands. We examined the surfaces at different time points by microscopy. We finally used confocal microscopy to observe DAPI-stained biofilms at high resolution.
Our results showed that biofilms grow to maximal confluency on plastic plates after 2 – 3 days of growth with daily exchange of the media, and patches of monolayer biofilms are clearly visible as early as 24 h.


Figure 1 and 2: Biofilm growth on filter paper at 10X magnification (Left), growth on filter paper at 20X magnification (Right).
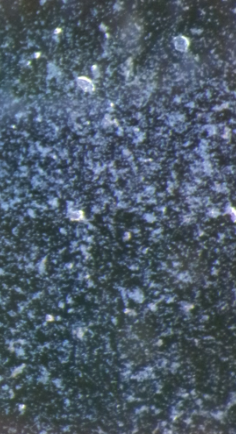

Figure 3 and 4: Biofilm growth on glass after 24 hours at 10X magnification (Left), growth on glass after 48 hours at 20X magnification (Right).


Figure 5 and 6: Biofilm growth on glass after 72 hours at 20X magnification (Left), growth on glass after 96 hours at 20X magnification (Right).


Figure 7 and 8: Biofilm growth on plastic after 24 hours at 20X magnification (Left), growth on plastic after 48 hours at 20X magnification (Right).


Figure 9 and 10: Biofilm growth on plastic after 72 hours at 10X magnification (Left), growth on plastic after 72 hours at 20X magnification (Right).

Figure 11: Biofilm growth on plastic after 96 hours at 20X magnification
Figure 12: Confocal image of DAPI stained 24 hour old biofilm showing patches of monolayer growth.
File:Movie.mov
Movie 1: 3D reconstruction of the DAPI stained biofilm Z-stacks.
We were therefore able to reproduce the published results of Caulobacter biofilm growth, and found an optimal set of growth conditions, times, and substratum surface for desirable biofilm formation.
What’s next? There are many aspects of the biofilm growth that need further improvement. First, in the final biological desalinator system, the biofilm grows on a membrane substratum that is permeable to ions and water. Guided by our preliminary results, we will seek the optimal membrane material for best biofilm growth.
Additionally, the desalinator biofilm should stay viable AND polarized for a long time in order to achieve maximal performance. This will be addressed by arresting the cell cycle using available simple techniques, such that the cells remain in the polarized state for an extended period of time. Finally, the biofilm should be sealed to prevent gradient-driven back-transport of the ions. This can be achieved automatically by the formation of tightly-packed monolayer biofilms, or by production of ‘sealing components’ at the lateral side of the cells to glue them together.
We also transformed the Caulobacter cells with integrating plasmids encoding inducible fluorescent proteins, as a practice for out later task of combining the pump/polarization modules with the biofilm module. The plasmids pXYFPC-2 and pVCFPC-4 were kindly provided by the Shapiro Lab at Stanford University. They encode YFP under a xylose-inducible promoter, and CFP under a vanillate-inducible promoter respectively.
References:
1. Ely, Bert. "[17] Genetics of Caulobacter crescentus." Methods in enzymology 204 (1991): 372-384.
2. Smit, John, Christopher S. Sherwood, and Robin FB Turner. "Characterization of high density monolayers of the biofilm bacterium Caulobacter crescentus: evaluating prospects for developing immobilized cell bioreactors." Canadian journal of microbiology 46.4 (2000): 339-349.
3. Entcheva-Dimitrov, Plamena, and Alfred M. Spormann. "Dynamics and control of biofilms of the oligotrophic bacterium Caulobacter crescentus." Journal of bacteriology 186.24 (2004): 8254-8266.
4. Bodenmiller, Diane, Evelyn Toh, and Yves V. Brun. "Development of surface adhesion in Caulobacter crescentus." Journal of bacteriology 186.5 (2004): 1438-1447.
 "
"