Template:Kyoto/Notebook/Aug 22
From 2013.igem.org
(Difference between revisions)
(→Restriction Enzyme Digestion) |
|||
| Line 110: | Line 110: | ||
|5min||30s||30s||1min||30cycles | |5min||30s||30s||1min||30cycles | ||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
</div> | </div> | ||
Revision as of 02:45, 25 September 2013
Contents |
Aug 22
Liquid culture
| Sample | Medium |
|---|---|
| 8/21 Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT (1) | 8/21 Plusgrow medium (+Amp) |
| 8/21 Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT (2) | 8/21 Plusgrow medium (+Amp) |
| 8/21 Pcon-RBS-GFP-DT control (1) | 8/21 Plusgrow medium (+Amp) |
| 8/21 Pcon-RBS-GFP-DT control (2) | 8/21 Plusgrow medium (+Amp) |
| 8/21 RBS-lysis1 (1) | 8/21 Plusgrow medium (+Amp) |
| 8/21 RBS-lysis1 (2) | 8/21 Plusgrow medium (+Amp) |
| 8/21 RBS control (1) | 8/21 Plusgrow medium (+Amp) |
| 8/21 RBS control (2) | 8/21 Plusgrow medium (+Amp) |
| 8/21 Ptet(pm)-RBS-tetR-DT (1) | 8/21 Plusgrow medium (+Amp) |
| 8/21 Ptet(pm)-RBS-tetR-DT (2) | 8/21 Plusgrow medium (+Amp) |
| 8/21 Ptet(pm) control (1) | 8/21 Plusgrow medium (+Amp) |
| 8/21 Ptet(pm) control (2) | 8/21 Plusgrow medium (+Amp) |
| 8/21 Plux-RBS-GFP-DT (1) | 8/21 Plusgrow medium (+Amp) |
| 8/21 pSB1C3(BBa_J04450) (1) | 8/21 Plusgrow medium (+Amp) |
| 8/21 pSB1C3(BBa_J04450) (2) | 8/21 Plusgrow medium (+Amp) |
incubate 37°C 10hour
Colony PCR
| Sample | base pair |
|---|---|
| 8/21 RBS-lysis1(1) | 400 |
| 8/21 RBS-lysis1(2) | 400 |
| 8/21 RBS control(1) | -- |
| 8/21 RBS control(2) | -- |
| negative control | -- |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 30s | 30cycles |
| Sample | base pair |
|---|---|
| 8/21 Pcon-GFP-DT-Pcon-RBS-luxR-DT (1) | 2143 |
| 8/21 Pcon-GFP-DT-Pcon-RBS-luxR-DT (2) | 2143 |
| 8/21 Pcon-RBS-luxR control(1) | -- |
| 8/21 Pcon-RBS-luxR control(2) | -- |
| negative control | -- |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 2min | 30cycles |
| Sample | base pair |
|---|---|
| 8/21 Ptet-RBS-tetR-DT (1) | 1216 |
| 8/21 Ptet-RBS-tetR-DT (2) | 1216 |
| 8/21 Ptet control (1) | -- |
| 8/21 Ptet control (2) | -- |
| 8/21 Plux-RBS-GFP-DT (1) | 1227 |
| 8/21 pSB1C3(BBa_J04450) (1) | 1353 |
| 8/21 pSB1C3(BBa_J04450) (2) | 1353 |
| negative control | -- |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 1min | 30cycles |
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 8/22 RBS-lysis (1) | 128 | 1.77 | 2.29 |
| 8/22 RBS-lysis (2) | 96 | 1.80 | 2.07 |
| 8/22 pSB1C3 (1) | 108 | 1.55 | 1.45 |
| 8/22 pSB1C3 (2) | 130 | 1.69 | 1.76 |
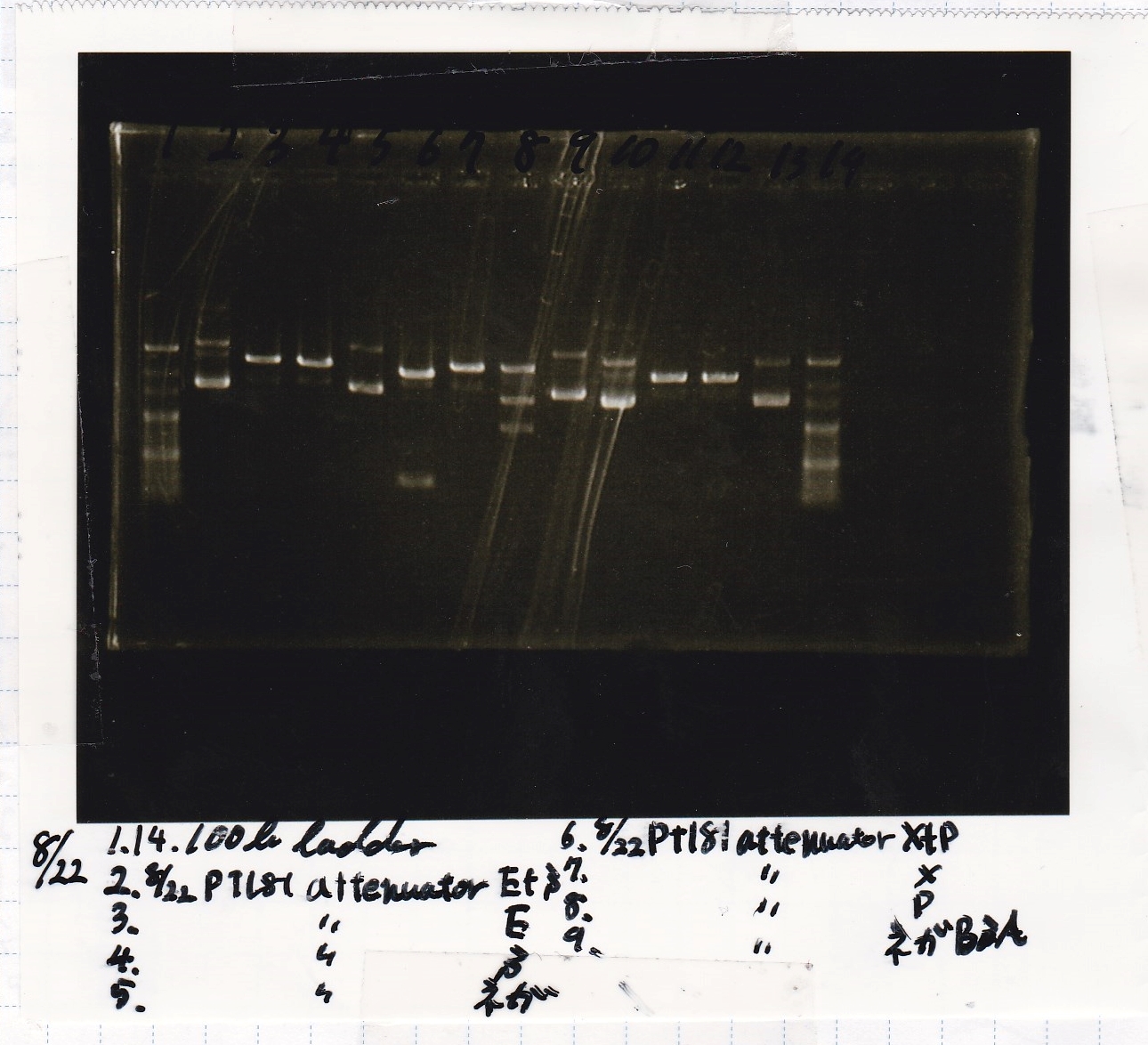
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp | -- | -- |
| 2 | 8/22 pT181 attenuator (1) | EcoRI | SpeI |
| 3 | 8/22 pT181 attenuator (1) | EcoRI | -- |
| 4 | 8/22 pT181 attenuator (1) | -- | SpeI |
| 5 | 8/22 pT181 attenuator (1) | -- | -- |
| 6 | 8/22 pT181 attenuator (1) | XbaI | PstI |
| 7 | 8/22 pT181 attenuator (1) | XbaI | -- |
| 8 | 8/22 pT181 attenuator (1) | -- | PstI |
| 9 | 8/22 pT181 attenuator (1) | -- | -- |
| 10 | 8/22 Fusion6 antisense (1) | EcoRI | SpeI |
| 11 | 8/22 Fusion6 antisense (1) | EcoRI | -- |
| 12 | 8/22 Fusion6 antisense (1) | -- | SpeI |
| 13 | 8/22 Fusion6 antisense (1) | -- | -- |
| 14 | 100bp | -- | -- |
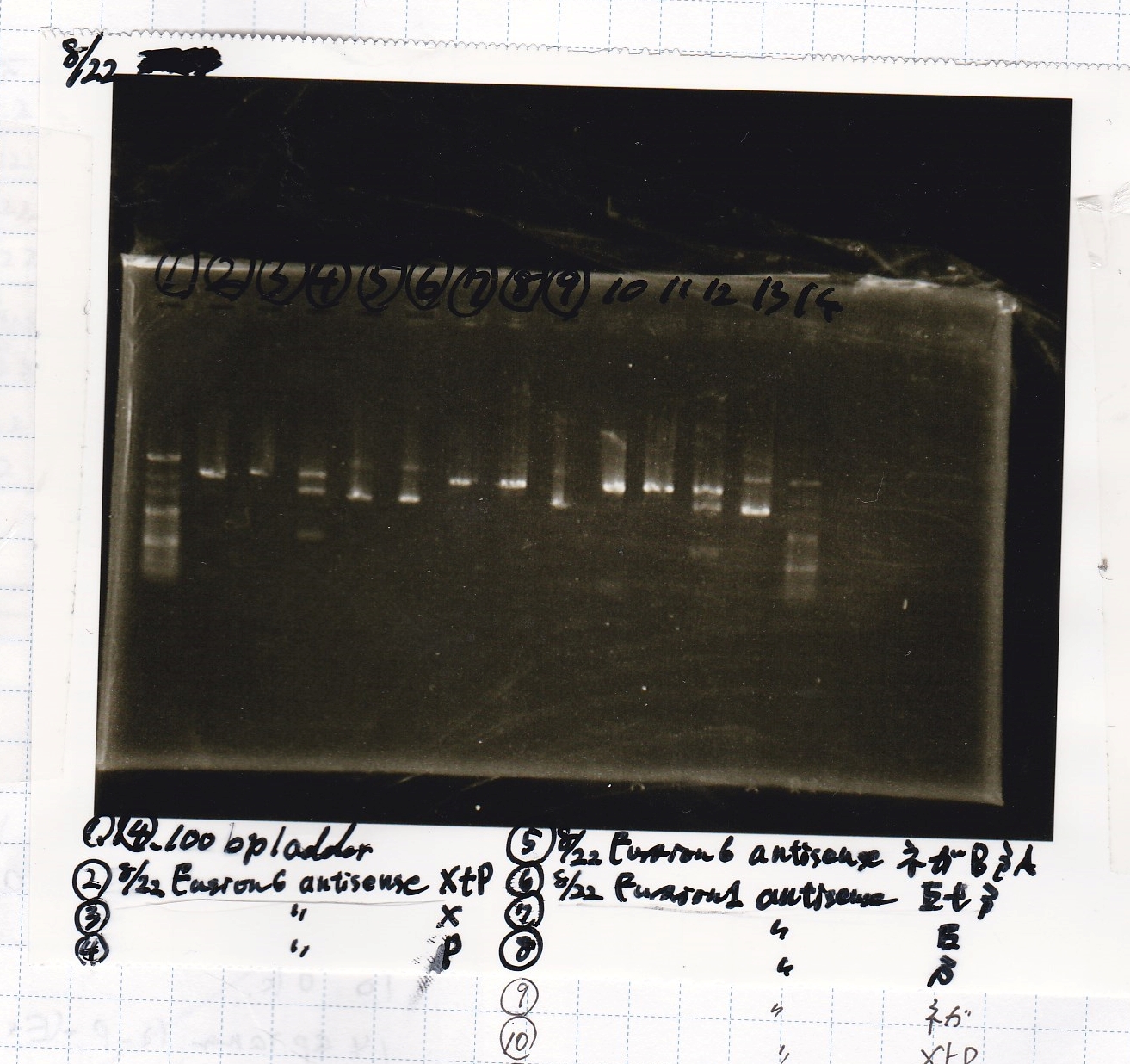
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp | -- | -- |
| 2 | 8/22 Fusion6 antisense (1) | XbaI | PstI |
| 3 | 8/22 Fusion6 antisense (1) | XbaI | -- |
| 4 | 8/22 Fusion6 antisense (1) | -- | PstI |
| 5 | 8/22 Fusion6 antisense (1) | -- | -- |
| 6 | 8/22 Fusion1 antisense (1) | EcoRI | SpeI |
| 7 | 8/22 Fusion1 antisense (1) | EcoRI | -- |
| 8 | 8/22 Fusion1 antisense (1) | -- | SpeI |
| 9 | 8/22 Fusion1 antisense (1) | -- | -- |
| 10 | 8/22 Fusion1 antisense (1) | XbaI | PstI |
| 11 | 8/22 Fusion1 antisense (1) | XbaI | -- |
| 12 | 8/22 Fusion1 antisense (1) | -- | PstI |
| 13 | 8/22 Fusion1 antisense (1) | -- | -- |
| 14 | 100bp | -- | -- |
 "
"