Template:Kyoto/Notebook/Aug 22
From 2013.igem.org
(Difference between revisions)
(Created page with "==Aug 22== ===Liquid culture=== <div class="experiment"> <span class="author">Nakamoto</span> {| class="wikitable" !|Sample||Medium |- |8/21 Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT (1)|...") |
(→Electrophoresis) |
||
| (18 intermediate revisions not shown) | |||
| Line 112: | Line 112: | ||
</div> | </div> | ||
| + | ===Restriction Enzyme Digestion=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Tatsui, Kojima, Honda and Ashida</span> | ||
| + | {| class="wikitable" | ||
| + | ! ||Fusion1 antisense (1) (300 µg/mL)||EcoRI||SpeI||buffer H||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||3.3||1||1||3||21.7||30 | ||
| + | |- | ||
| + | |1 cut||0.7||0.2||0||1||8.1||10 | ||
| + | |- | ||
| + | |1 cut||0.7||0||0.2||1||8.1||10 | ||
| + | |- | ||
| + | |NC||0.7||0||0||1||8.3||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||Fusion1 antisense (1) (300 µg/mL)||XbaI||PstI||buffer M||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||3.3||1||1||3||3||18.7||30 | ||
| + | |- | ||
| + | |1 cut||0.7||0.2||0||1||1||7.1||10 | ||
| + | |- | ||
| + | |1 cut||0.7||0||0.2||1||1||7.1||10 | ||
| + | |- | ||
| + | |NC||0.7||0||0||1||1||7.3||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||Fusion6 antisense (1) (336 µg/mL)||EcoRI||SpeI||buffer H||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||3||1||1||3||22||30 | ||
| + | |- | ||
| + | |1 cut||0.6||0.2||0||1||8.2||10 | ||
| + | |- | ||
| + | |1 cut||0.6||0||0.2||1||8.2||10 | ||
| + | |- | ||
| + | |NC||0.6||0||0||1||8.4||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||Fusion6 antisense (1) (336 µg/mL)||XbaI||PstI||buffer M||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||3||1||1||3||3||19||30 | ||
| + | |- | ||
| + | |1 cut||0.6||0.2||0||1||1||7.2||10 | ||
| + | |- | ||
| + | |1 cut||0.6||0||0.2||1||1||7.2||10 | ||
| + | |- | ||
| + | |NC||0.6||0||0||1||1||7.4||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||pT181 antisense (1) (178 µg/mL)||EcoRI||SpeI||buffer H||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||5.6||1||1||3||19.4||30 | ||
| + | |- | ||
| + | |1 cut||1.1||0.2||0||1||7.7||10 | ||
| + | |- | ||
| + | |1 cut||1.1||0||0.2||1||7.7||10 | ||
| + | |- | ||
| + | |NC||1.1||0||0||1||7.9||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||pT181 antisense (1) (178 µg/mL)||XbaI||PstI||buffer M||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||5.6||1||1||3||3||16.4||30 | ||
| + | |- | ||
| + | |1 cut||1.1||0.2||0||1||1||6.7||10 | ||
| + | |- | ||
| + | |1 cut||1.1||0||0.2||1||1||6.7||10 | ||
| + | |- | ||
| + | |NC||1.1||0||0||1||1||6.9||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||pT181 attenuator (1)||EcoRI||SpeI||buffer H||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||3||1||1||3||22||30 | ||
| + | |- | ||
| + | |1 cut||0.6||0.2||0||1||8.2||10 | ||
| + | |- | ||
| + | |1 cut||0.6||0||0.2||1||8.2||10 | ||
| + | |- | ||
| + | |NC||0.6||0||0||1||8.4||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||pT181 attenuator (1)||XbaI||PstI||buffer M||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||3||1||1||3||3||19||30 | ||
| + | |- | ||
| + | |1 cut||0.6||0.2||0||1||1||7.2||10 | ||
| + | |- | ||
| + | |1 cut||0.6||0||0.2||1||1||7.2||10 | ||
| + | |- | ||
| + | |NC||0.6||0||0||1||1||7.4||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||Fusion1 attenuator (1)||EcoRI||SpeI||buffer H||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||3.3||1||1||3||21.7||30 | ||
| + | |- | ||
| + | |1 cut||0.7||0.2||0||1||8.1||10 | ||
| + | |- | ||
| + | |1 cut||0.7||0||0.2||1||8.1||10 | ||
| + | |- | ||
| + | |NC||0.7||0||0||1||8.3||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||Fusion1 attenuator (1)||XbaI||PstI||buffer M||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||3.3||1||1||3||3||18.7||30 | ||
| + | |- | ||
| + | |1 cut||0.7||0.2||0||1||1||7.1||10 | ||
| + | |- | ||
| + | |1 cut||0.7||0||0.2||1||1||7.1||10 | ||
| + | |- | ||
| + | |NC||0.7||0||0||1||1||7.3||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||Fusion3m2 attenuator (1)||EcoRI||SpeI||buffer H||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||3||1||1||3||22||30 | ||
| + | |- | ||
| + | |1 cut||0.6||0.2||0||1||8.2||10 | ||
| + | |- | ||
| + | |1 cut||0.6||0||0.2||1||8.2||10 | ||
| + | |- | ||
| + | |NC||0.6||0||0||1||8.4||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||Fusion3m2 attenuator (1)||XbaI||PstI||buffer M||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||3||1||1||3||3||19||30 | ||
| + | |- | ||
| + | |1 cut||0.6||0.2||0||1||1||7.2||10 | ||
| + | |- | ||
| + | |1 cut||0.6||0||0.2||1||1||7.2||10 | ||
| + | |- | ||
| + | |NC||0.6||0||0||1||1||7.4||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||Spinach (2)||EcoRI||SpeI||buffer B||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||2.3||0.5||0.5||3||0.3||23.4||30 | ||
| + | |- | ||
| + | |1 cut||0.5||0.1||0||1||0.1||8.3||10 | ||
| + | |- | ||
| + | |1 cut||0.5||0||0.1||1||0.1||8.3||10 | ||
| + | |- | ||
| + | |NC||0.5||0||0||1||0.1||8.4||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||tetR aptamer 12_P (2)||EcoRI||SpeI||buffer B||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||2.7||0.5||0.5||3||0.3||23||30 | ||
| + | |- | ||
| + | |1 cut||0.5||0.1||0||1||0.1||8.3||10 | ||
| + | |- | ||
| + | |1 cut||0.5||0||0.1||1||0.1||8.3||10 | ||
| + | |- | ||
| + | |NC||0.5||0||0||1||0.1||8.4||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||tetR aptamer 12_1R (2)||EcoRI||SpeI||buffer B||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||2.6||0.5||0.5||3||0.3||23.1||30 | ||
| + | |- | ||
| + | |1 cut||0.5||0.1||0||1||0.1||8.3||10 | ||
| + | |- | ||
| + | |1 cut||0.5||0||0.1||1||0.1||8.3||10 | ||
| + | |- | ||
| + | |NC||0.5||0||0||1||0.1||8.4||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||tetR aptamer 12_1M (1)||EcoRI||SpeI||buffer B||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||2.7||0.5||0.5||3||0.3||23||30 | ||
| + | |- | ||
| + | |1 cut||0.5||0.1||0||1||0.1||8.3||10 | ||
| + | |- | ||
| + | |1 cut||0.5||0||0.1||1||0.1||8.3||10 | ||
| + | |- | ||
| + | |NC||0.5||0||0||1||0.1||8.4||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||J23100||SpeI||PstI||buffer B||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||7||0.5||0.5||3||0.3||18.7||30 | ||
| + | |- | ||
| + | |1 cut||1.4||0.1||0||1||0.1||7.4||10 | ||
| + | |- | ||
| + | |1 cut||1.4||0||0.1||1||0.1||7.4||10 | ||
| + | |- | ||
| + | |NC||1.4||0||0||1||0.1||7.5||10 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||Plac (2)||SpeI||PstI||buffer B||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||11.2||0.5||0.5||3||0.3||14.5||30 | ||
| + | |- | ||
| + | |1 cut||2.2||0.1||0||1||0.1||6.6||10 | ||
| + | |- | ||
| + | |1 cut||2.2||0||0.1||1||0.1||6.6||10 | ||
| + | |- | ||
| + | |NC||2.2||0||0||1||0.1||6.7||10 | ||
| + | |} | ||
| + | </div> | ||
===Electrophoresis=== | ===Electrophoresis=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">No name</span> | <span class="author">No name</span> | ||
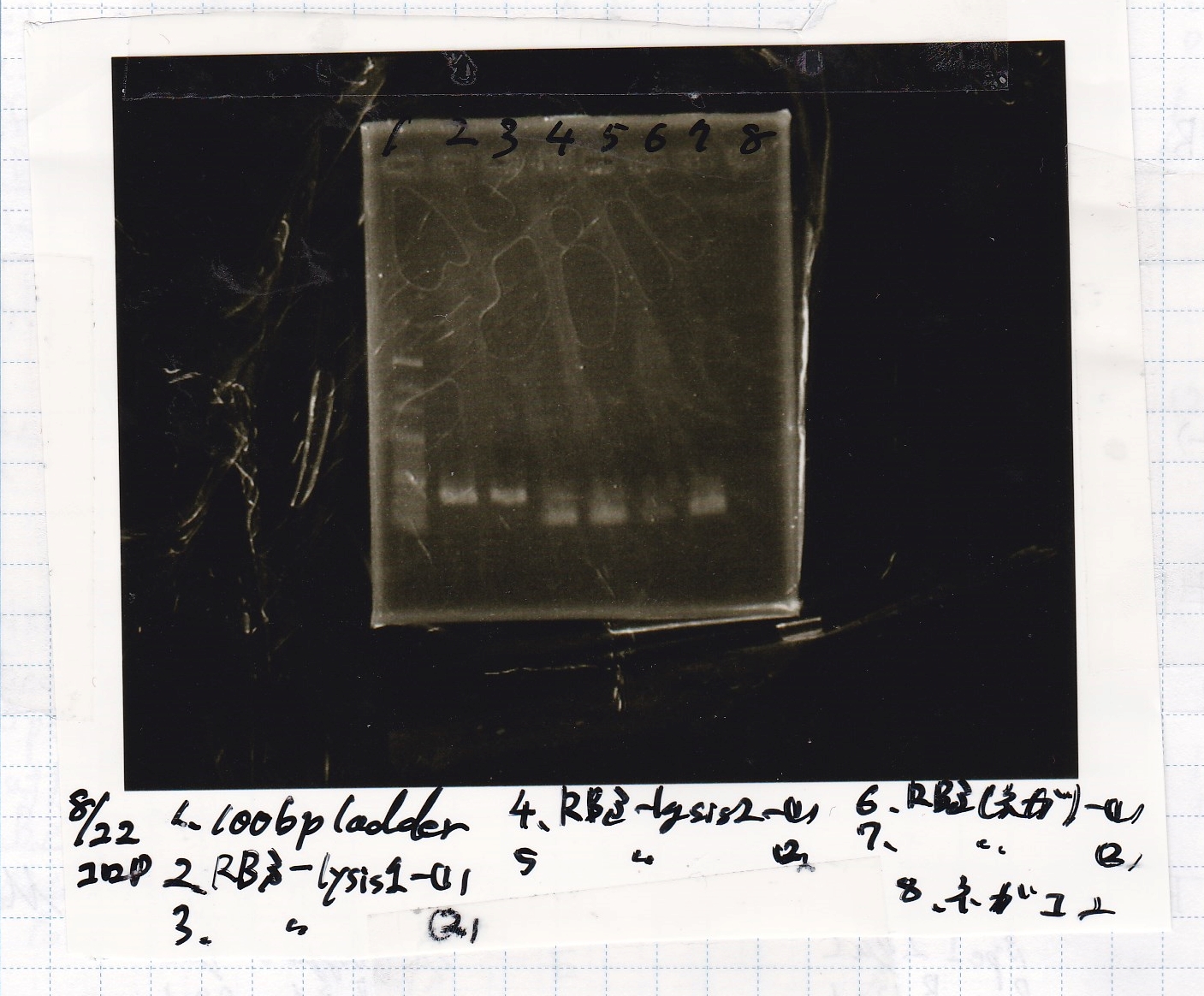
{| class="wikitable" | {| class="wikitable" | ||
| - | !Lane||Sample | + | !Lane||Sample |
|- | |- | ||
| - | |1|| | + | |1||100bp ladder |
|- | |- | ||
| - | |2|| | + | |2||RBS-lysis1 -(1) |
|- | |- | ||
| - | |3|| | + | |3||RBS-lysis1 -(2) |
|- | |- | ||
| - | |4|| | + | |4||RBS-lysis2 -(1) |
|- | |- | ||
| - | |5|| | + | |5||RBS-lysis2 -(2) |
|- | |- | ||
| - | |6|| | + | |6||RBS(NC) -(1) |
|- | |- | ||
| - | |7|| | + | |7||RBS(NC) -(2) |
|- | |- | ||
| - | |8|| | + | |8||NC |
| + | |} | ||
| + | [[File:Igku Aug22 Electrophoresis(ColoP)-1.jpg]]<br> | ||
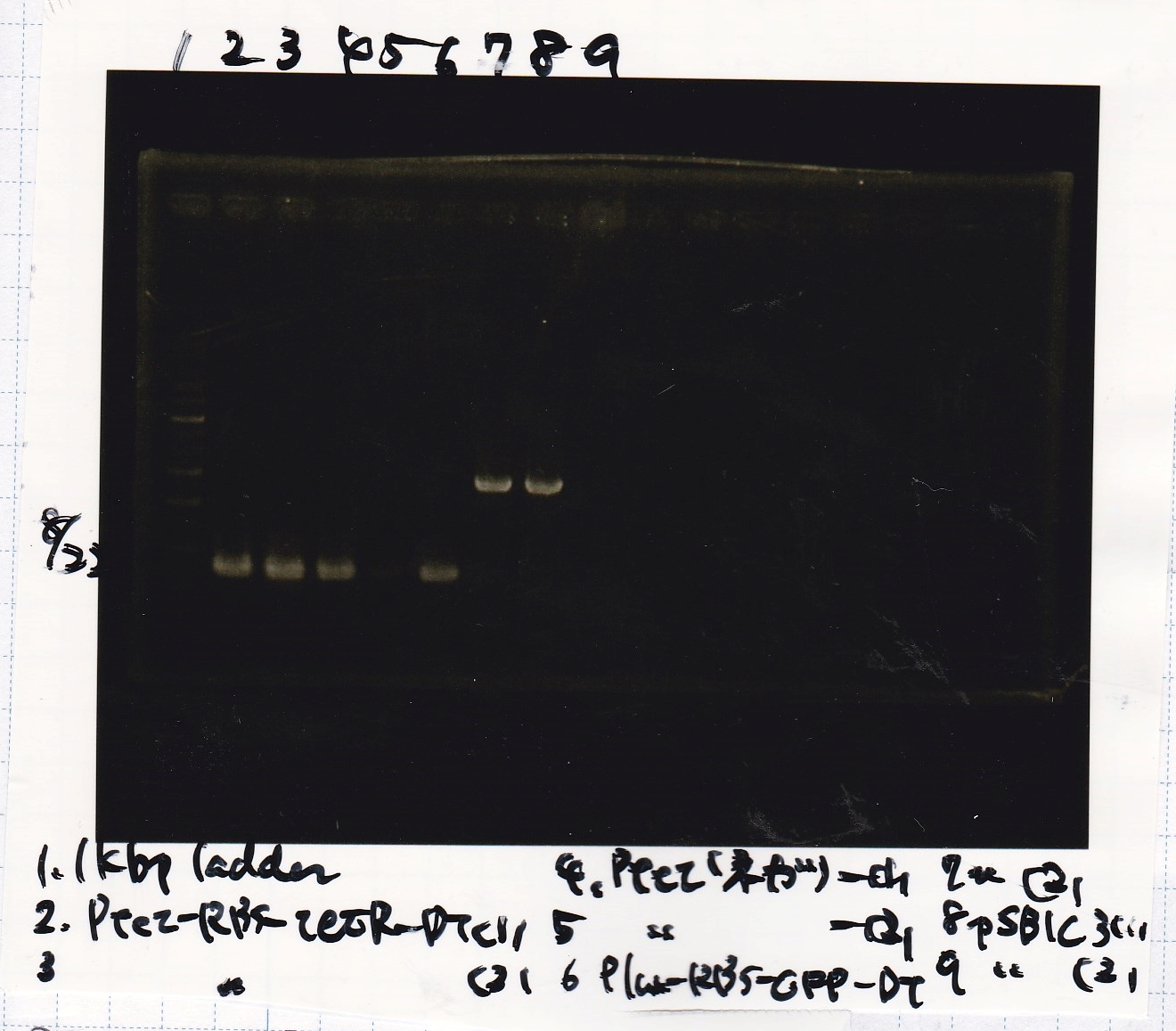
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
|- | |- | ||
| - | | | + | |1||1kb ladder |
|- | |- | ||
| - | | | + | |2||Ptet-RBS-tetR-DT -(1) |
|- | |- | ||
| - | | | + | |3||Ptet-RBS-tetR-DT -(2) |
|- | |- | ||
| - | | | + | |4||Ptet(NC) -(1) |
|- | |- | ||
| - | | | + | |5||Ptet(NC) -(2) |
|- | |- | ||
| - | | | + | |6||Plux-RBS-GFP-DT -(1) |
|- | |- | ||
| - | | | + | |7||pSB1C3 -(1) |
|- | |- | ||
| - | | | + | |8||pSB1C3 -(2) |
|- | |- | ||
| - | | | + | |9||NC |
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug22 Electrophoresis(ColoP)-2.jpg]]<br> |
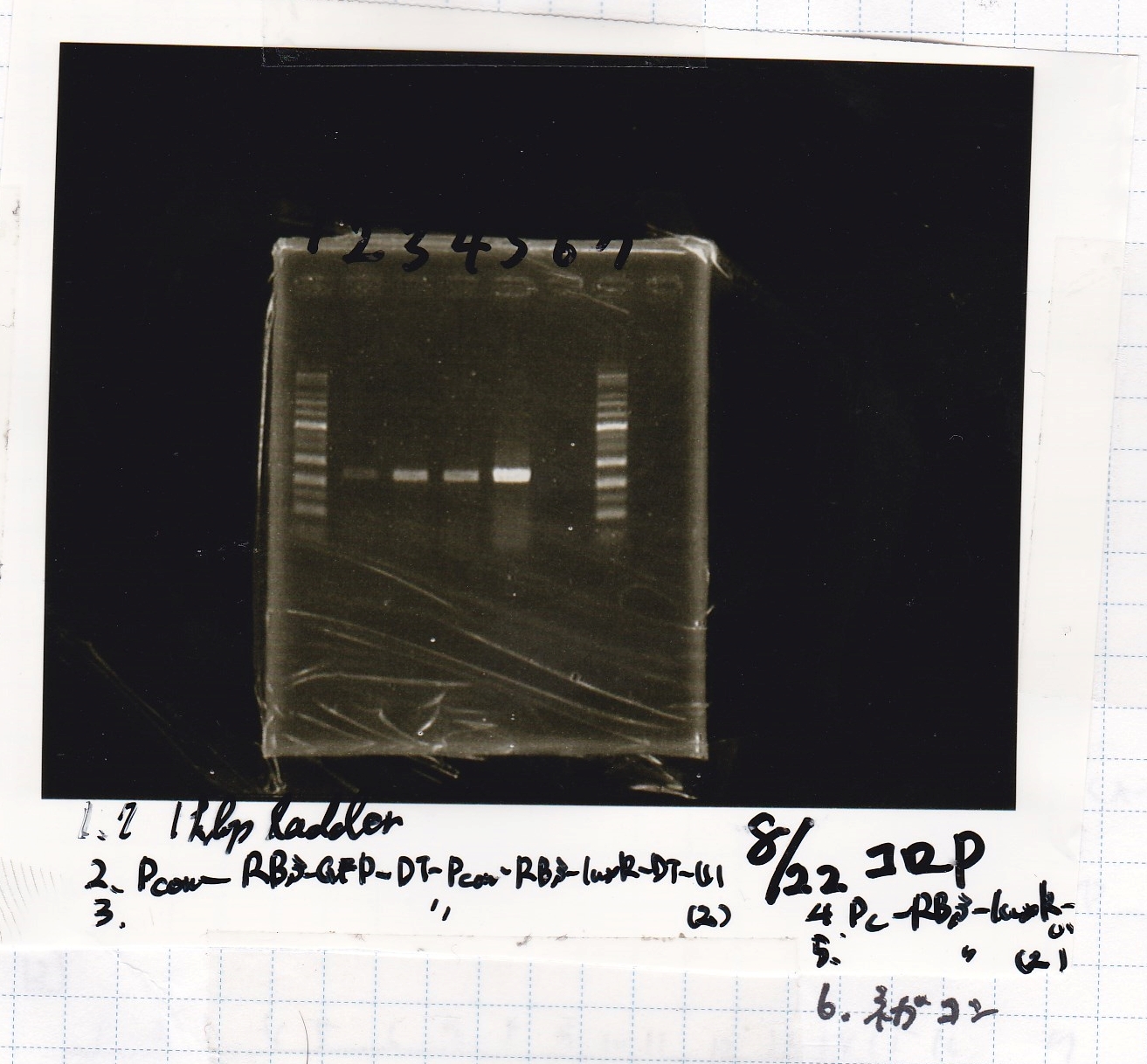
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||1kb ladder | ||
| + | |- | ||
| + | |2||Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT -(1) | ||
| + | |- | ||
| + | |3||Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT -(2) | ||
| + | |- | ||
| + | |4||Pcon-RBS-luxR -(1) | ||
| + | |- | ||
| + | |5||Pcon-RBS-luxR -(2) | ||
| + | |- | ||
| + | |6||NC | ||
| + | |- | ||
| + | |7||1kb ladder | ||
| + | |} | ||
| + | [[File:Igku Aug22 Electrophoresis(ColoP)-3.jpg]]<br> | ||
| + | </div> | ||
| + | ===Miniprep=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Ashida</span> | ||
| + | {|class="wikitable" | ||
| + | !DNA||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |8/22 RBS-lysis (1)||128||1.77||2.29 | ||
| + | |- | ||
| + | |8/22 RBS-lysis (2)||96||1.80||2.07 | ||
| + | |- | ||
| + | |8/22 pSB1C3 (1)||108||1.55||1.45 | ||
| + | |- | ||
| + | |8/22 pSB1C3 (2)||130||1.69||1.76 | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Electrophoresis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
{| class="wikitable" | {| class="wikitable" | ||
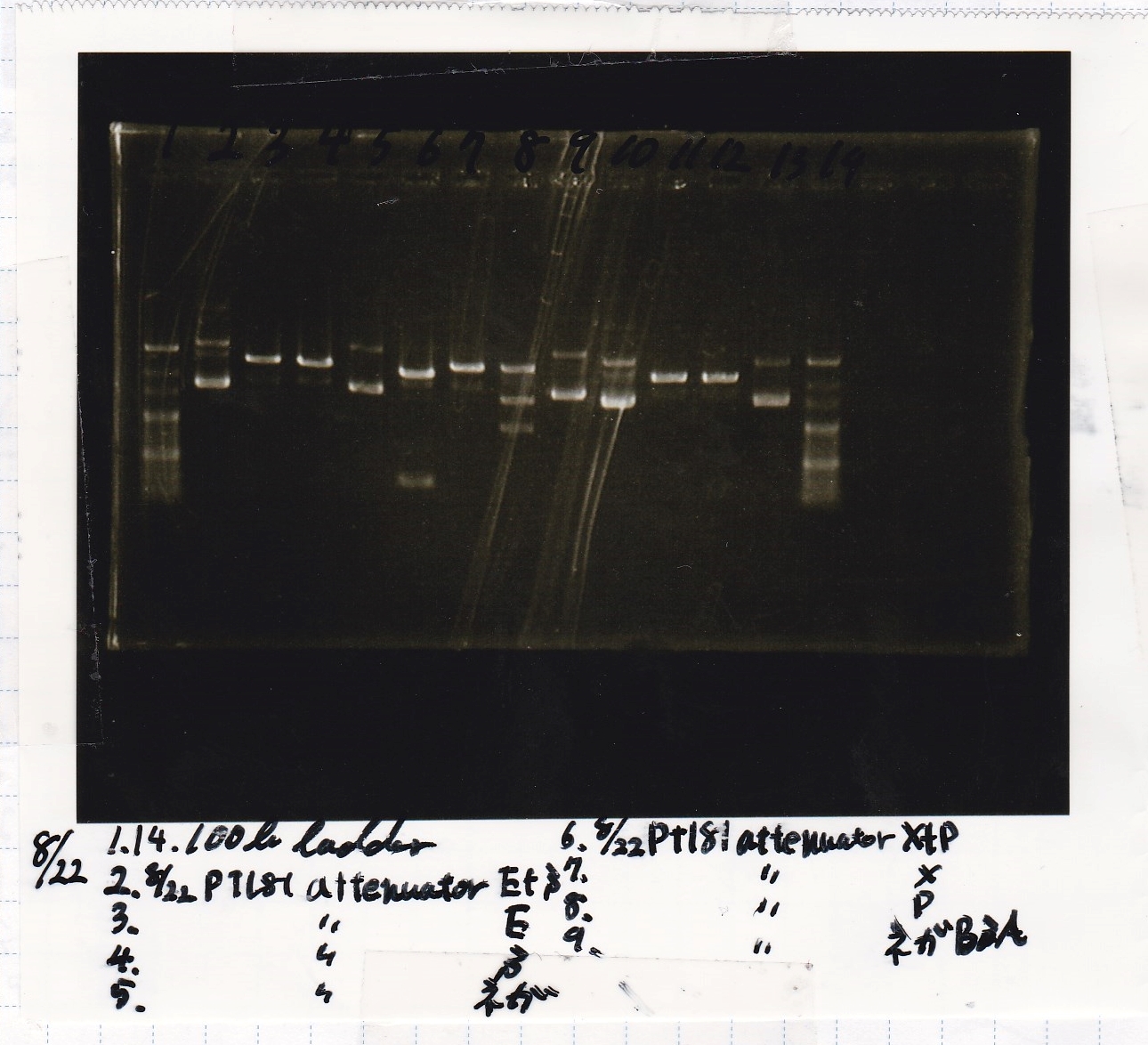
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
|- | |- | ||
| - | |1|| | + | |1||100bp||--||-- |
|- | |- | ||
| - | |2|| | + | |2||8/22 pT181 attenuator (1)||EcoRI||SpeI |
|- | |- | ||
| - | |3|| | + | |3||8/22 pT181 attenuator (1)||EcoRI||-- |
|- | |- | ||
| - | |4|| | + | |4||8/22 pT181 attenuator (1)||--||SpeI |
|- | |- | ||
| - | |5|| | + | |5||8/22 pT181 attenuator (1)||--||-- |
|- | |- | ||
| - | |6|| | + | |6||8/22 pT181 attenuator (1)||XbaI||PstI |
|- | |- | ||
| - | |7|| | + | |7||8/22 pT181 attenuator (1)||XbaI||-- |
|- | |- | ||
| - | |8|| | + | |8||8/22 pT181 attenuator (1)||--||PstI |
|- | |- | ||
| - | |9|| | + | |9||8/22 pT181 attenuator (1)||--||-- |
|- | |- | ||
| - | |10|| | + | |10||8/22 Fusion6 antisense (1)||EcoRI||SpeI |
|- | |- | ||
| - | |11|| | + | |11||8/22 Fusion6 antisense (1)||EcoRI||-- |
|- | |- | ||
| - | |12|| | + | |12||8/22 Fusion6 antisense (1)||--||SpeI |
|- | |- | ||
| - | |13|| | + | |13||8/22 Fusion6 antisense (1)||--||-- |
|- | |- | ||
| - | |14|| | + | |14||100bp||--||-- |
| + | |} | ||
| + | [[File:Igku_Aug22electrophoresis.jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||100bp||--||-- | ||
| + | |- | ||
| + | |2||8/22 Fusion6 antisense (1)||XbaI||PstI | ||
| + | |- | ||
| + | |3||8/22 Fusion6 antisense (1)||XbaI||-- | ||
| + | |- | ||
| + | |4||8/22 Fusion6 antisense (1)||--||PstI | ||
| + | |- | ||
| + | |5||8/22 Fusion6 antisense (1)||--||-- | ||
| + | |- | ||
| + | |6||8/22 Fusion1 antisense (1)||EcoRI||SpeI | ||
| + | |- | ||
| + | |7||8/22 Fusion1 antisense (1)||EcoRI||-- | ||
| + | |- | ||
| + | |8||8/22 Fusion1 antisense (1)||--||SpeI | ||
| + | |- | ||
| + | |9||8/22 Fusion1 antisense (1)||--||-- | ||
| + | |- | ||
| + | |10||8/22 Fusion1 antisense (1)||XbaI||PstI | ||
| + | |- | ||
| + | |11||8/22 Fusion1 antisense (1)||XbaI||-- | ||
|- | |- | ||
| - | | | + | |12||8/22 Fusion1 antisense (1)||--||PstI |
|- | |- | ||
| - | | | + | |13||8/22 Fusion1 antisense (1)||--||-- |
|- | |- | ||
| - | | | + | |14||100bp||--||-- |
|} | |} | ||
| - | [[File: | + | [[File:Igku_Aug22electrophoresis2.jpg]]<br> |
</div> | </div> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Latest revision as of 14:49, 25 September 2013
Contents |
Aug 22
Liquid culture
| Sample | Medium |
|---|---|
| 8/21 Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT (1) | 8/21 Plusgrow medium (+Amp) |
| 8/21 Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT (2) | 8/21 Plusgrow medium (+Amp) |
| 8/21 Pcon-RBS-GFP-DT control (1) | 8/21 Plusgrow medium (+Amp) |
| 8/21 Pcon-RBS-GFP-DT control (2) | 8/21 Plusgrow medium (+Amp) |
| 8/21 RBS-lysis1 (1) | 8/21 Plusgrow medium (+Amp) |
| 8/21 RBS-lysis1 (2) | 8/21 Plusgrow medium (+Amp) |
| 8/21 RBS control (1) | 8/21 Plusgrow medium (+Amp) |
| 8/21 RBS control (2) | 8/21 Plusgrow medium (+Amp) |
| 8/21 Ptet(pm)-RBS-tetR-DT (1) | 8/21 Plusgrow medium (+Amp) |
| 8/21 Ptet(pm)-RBS-tetR-DT (2) | 8/21 Plusgrow medium (+Amp) |
| 8/21 Ptet(pm) control (1) | 8/21 Plusgrow medium (+Amp) |
| 8/21 Ptet(pm) control (2) | 8/21 Plusgrow medium (+Amp) |
| 8/21 Plux-RBS-GFP-DT (1) | 8/21 Plusgrow medium (+Amp) |
| 8/21 pSB1C3(BBa_J04450) (1) | 8/21 Plusgrow medium (+Amp) |
| 8/21 pSB1C3(BBa_J04450) (2) | 8/21 Plusgrow medium (+Amp) |
incubate 37°C 10hour
Colony PCR
| Sample | base pair |
|---|---|
| 8/21 RBS-lysis1(1) | 400 |
| 8/21 RBS-lysis1(2) | 400 |
| 8/21 RBS control(1) | -- |
| 8/21 RBS control(2) | -- |
| negative control | -- |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 30s | 30cycles |
| Sample | base pair |
|---|---|
| 8/21 Pcon-GFP-DT-Pcon-RBS-luxR-DT (1) | 2143 |
| 8/21 Pcon-GFP-DT-Pcon-RBS-luxR-DT (2) | 2143 |
| 8/21 Pcon-RBS-luxR control(1) | -- |
| 8/21 Pcon-RBS-luxR control(2) | -- |
| negative control | -- |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 2min | 30cycles |
| Sample | base pair |
|---|---|
| 8/21 Ptet-RBS-tetR-DT (1) | 1216 |
| 8/21 Ptet-RBS-tetR-DT (2) | 1216 |
| 8/21 Ptet control (1) | -- |
| 8/21 Ptet control (2) | -- |
| 8/21 Plux-RBS-GFP-DT (1) | 1227 |
| 8/21 pSB1C3(BBa_J04450) (1) | 1353 |
| 8/21 pSB1C3(BBa_J04450) (2) | 1353 |
| negative control | -- |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 1min | 30cycles |
Restriction Enzyme Digestion
| Fusion1 antisense (1) (300 µg/mL) | EcoRI | SpeI | buffer H | MilliQ | total | |
|---|---|---|---|---|---|---|
| 2 cuts | 3.3 | 1 | 1 | 3 | 21.7 | 30 |
| 1 cut | 0.7 | 0.2 | 0 | 1 | 8.1 | 10 |
| 1 cut | 0.7 | 0 | 0.2 | 1 | 8.1 | 10 |
| NC | 0.7 | 0 | 0 | 1 | 8.3 | 10 |
| Fusion1 antisense (1) (300 µg/mL) | XbaI | PstI | buffer M | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 3.3 | 1 | 1 | 3 | 3 | 18.7 | 30 |
| 1 cut | 0.7 | 0.2 | 0 | 1 | 1 | 7.1 | 10 |
| 1 cut | 0.7 | 0 | 0.2 | 1 | 1 | 7.1 | 10 |
| NC | 0.7 | 0 | 0 | 1 | 1 | 7.3 | 10 |
| Fusion6 antisense (1) (336 µg/mL) | EcoRI | SpeI | buffer H | MilliQ | total | |
|---|---|---|---|---|---|---|
| 2 cuts | 3 | 1 | 1 | 3 | 22 | 30 |
| 1 cut | 0.6 | 0.2 | 0 | 1 | 8.2 | 10 |
| 1 cut | 0.6 | 0 | 0.2 | 1 | 8.2 | 10 |
| NC | 0.6 | 0 | 0 | 1 | 8.4 | 10 |
| Fusion6 antisense (1) (336 µg/mL) | XbaI | PstI | buffer M | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 3 | 1 | 1 | 3 | 3 | 19 | 30 |
| 1 cut | 0.6 | 0.2 | 0 | 1 | 1 | 7.2 | 10 |
| 1 cut | 0.6 | 0 | 0.2 | 1 | 1 | 7.2 | 10 |
| NC | 0.6 | 0 | 0 | 1 | 1 | 7.4 | 10 |
| pT181 antisense (1) (178 µg/mL) | EcoRI | SpeI | buffer H | MilliQ | total | |
|---|---|---|---|---|---|---|
| 2 cuts | 5.6 | 1 | 1 | 3 | 19.4 | 30 |
| 1 cut | 1.1 | 0.2 | 0 | 1 | 7.7 | 10 |
| 1 cut | 1.1 | 0 | 0.2 | 1 | 7.7 | 10 |
| NC | 1.1 | 0 | 0 | 1 | 7.9 | 10 |
| pT181 antisense (1) (178 µg/mL) | XbaI | PstI | buffer M | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 5.6 | 1 | 1 | 3 | 3 | 16.4 | 30 |
| 1 cut | 1.1 | 0.2 | 0 | 1 | 1 | 6.7 | 10 |
| 1 cut | 1.1 | 0 | 0.2 | 1 | 1 | 6.7 | 10 |
| NC | 1.1 | 0 | 0 | 1 | 1 | 6.9 | 10 |
| pT181 attenuator (1) | EcoRI | SpeI | buffer H | MilliQ | total | |
|---|---|---|---|---|---|---|
| 2 cuts | 3 | 1 | 1 | 3 | 22 | 30 |
| 1 cut | 0.6 | 0.2 | 0 | 1 | 8.2 | 10 |
| 1 cut | 0.6 | 0 | 0.2 | 1 | 8.2 | 10 |
| NC | 0.6 | 0 | 0 | 1 | 8.4 | 10 |
| pT181 attenuator (1) | XbaI | PstI | buffer M | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 3 | 1 | 1 | 3 | 3 | 19 | 30 |
| 1 cut | 0.6 | 0.2 | 0 | 1 | 1 | 7.2 | 10 |
| 1 cut | 0.6 | 0 | 0.2 | 1 | 1 | 7.2 | 10 |
| NC | 0.6 | 0 | 0 | 1 | 1 | 7.4 | 10 |
| Fusion1 attenuator (1) | EcoRI | SpeI | buffer H | MilliQ | total | |
|---|---|---|---|---|---|---|
| 2 cuts | 3.3 | 1 | 1 | 3 | 21.7 | 30 |
| 1 cut | 0.7 | 0.2 | 0 | 1 | 8.1 | 10 |
| 1 cut | 0.7 | 0 | 0.2 | 1 | 8.1 | 10 |
| NC | 0.7 | 0 | 0 | 1 | 8.3 | 10 |
| Fusion1 attenuator (1) | XbaI | PstI | buffer M | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 3.3 | 1 | 1 | 3 | 3 | 18.7 | 30 |
| 1 cut | 0.7 | 0.2 | 0 | 1 | 1 | 7.1 | 10 |
| 1 cut | 0.7 | 0 | 0.2 | 1 | 1 | 7.1 | 10 |
| NC | 0.7 | 0 | 0 | 1 | 1 | 7.3 | 10 |
| Fusion3m2 attenuator (1) | EcoRI | SpeI | buffer H | MilliQ | total | |
|---|---|---|---|---|---|---|
| 2 cuts | 3 | 1 | 1 | 3 | 22 | 30 |
| 1 cut | 0.6 | 0.2 | 0 | 1 | 8.2 | 10 |
| 1 cut | 0.6 | 0 | 0.2 | 1 | 8.2 | 10 |
| NC | 0.6 | 0 | 0 | 1 | 8.4 | 10 |
| Fusion3m2 attenuator (1) | XbaI | PstI | buffer M | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 3 | 1 | 1 | 3 | 3 | 19 | 30 |
| 1 cut | 0.6 | 0.2 | 0 | 1 | 1 | 7.2 | 10 |
| 1 cut | 0.6 | 0 | 0.2 | 1 | 1 | 7.2 | 10 |
| NC | 0.6 | 0 | 0 | 1 | 1 | 7.4 | 10 |
| Spinach (2) | EcoRI | SpeI | buffer B | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 2.3 | 0.5 | 0.5 | 3 | 0.3 | 23.4 | 30 |
| 1 cut | 0.5 | 0.1 | 0 | 1 | 0.1 | 8.3 | 10 |
| 1 cut | 0.5 | 0 | 0.1 | 1 | 0.1 | 8.3 | 10 |
| NC | 0.5 | 0 | 0 | 1 | 0.1 | 8.4 | 10 |
| tetR aptamer 12_P (2) | EcoRI | SpeI | buffer B | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 2.7 | 0.5 | 0.5 | 3 | 0.3 | 23 | 30 |
| 1 cut | 0.5 | 0.1 | 0 | 1 | 0.1 | 8.3 | 10 |
| 1 cut | 0.5 | 0 | 0.1 | 1 | 0.1 | 8.3 | 10 |
| NC | 0.5 | 0 | 0 | 1 | 0.1 | 8.4 | 10 |
| tetR aptamer 12_1R (2) | EcoRI | SpeI | buffer B | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 2.6 | 0.5 | 0.5 | 3 | 0.3 | 23.1 | 30 |
| 1 cut | 0.5 | 0.1 | 0 | 1 | 0.1 | 8.3 | 10 |
| 1 cut | 0.5 | 0 | 0.1 | 1 | 0.1 | 8.3 | 10 |
| NC | 0.5 | 0 | 0 | 1 | 0.1 | 8.4 | 10 |
| tetR aptamer 12_1M (1) | EcoRI | SpeI | buffer B | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 2.7 | 0.5 | 0.5 | 3 | 0.3 | 23 | 30 |
| 1 cut | 0.5 | 0.1 | 0 | 1 | 0.1 | 8.3 | 10 |
| 1 cut | 0.5 | 0 | 0.1 | 1 | 0.1 | 8.3 | 10 |
| NC | 0.5 | 0 | 0 | 1 | 0.1 | 8.4 | 10 |
| J23100 | SpeI | PstI | buffer B | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 7 | 0.5 | 0.5 | 3 | 0.3 | 18.7 | 30 |
| 1 cut | 1.4 | 0.1 | 0 | 1 | 0.1 | 7.4 | 10 |
| 1 cut | 1.4 | 0 | 0.1 | 1 | 0.1 | 7.4 | 10 |
| NC | 1.4 | 0 | 0 | 1 | 0.1 | 7.5 | 10 |
| Plac (2) | SpeI | PstI | buffer B | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 11.2 | 0.5 | 0.5 | 3 | 0.3 | 14.5 | 30 |
| 1 cut | 2.2 | 0.1 | 0 | 1 | 0.1 | 6.6 | 10 |
| 1 cut | 2.2 | 0 | 0.1 | 1 | 0.1 | 6.6 | 10 |
| NC | 2.2 | 0 | 0 | 1 | 0.1 | 6.7 | 10 |
Electrophoresis
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | RBS-lysis1 -(1) |
| 3 | RBS-lysis1 -(2) |
| 4 | RBS-lysis2 -(1) |
| 5 | RBS-lysis2 -(2) |
| 6 | RBS(NC) -(1) |
| 7 | RBS(NC) -(2) |
| 8 | NC |
| Lane | Sample |
|---|---|
| 1 | 1kb ladder |
| 2 | Ptet-RBS-tetR-DT -(1) |
| 3 | Ptet-RBS-tetR-DT -(2) |
| 4 | Ptet(NC) -(1) |
| 5 | Ptet(NC) -(2) |
| 6 | Plux-RBS-GFP-DT -(1) |
| 7 | pSB1C3 -(1) |
| 8 | pSB1C3 -(2) |
| 9 | NC |
| Lane | Sample |
|---|---|
| 1 | 1kb ladder |
| 2 | Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT -(1) |
| 3 | Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT -(2) |
| 4 | Pcon-RBS-luxR -(1) |
| 5 | Pcon-RBS-luxR -(2) |
| 6 | NC |
| 7 | 1kb ladder |
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 8/22 RBS-lysis (1) | 128 | 1.77 | 2.29 |
| 8/22 RBS-lysis (2) | 96 | 1.80 | 2.07 |
| 8/22 pSB1C3 (1) | 108 | 1.55 | 1.45 |
| 8/22 pSB1C3 (2) | 130 | 1.69 | 1.76 |
Electrophoresis
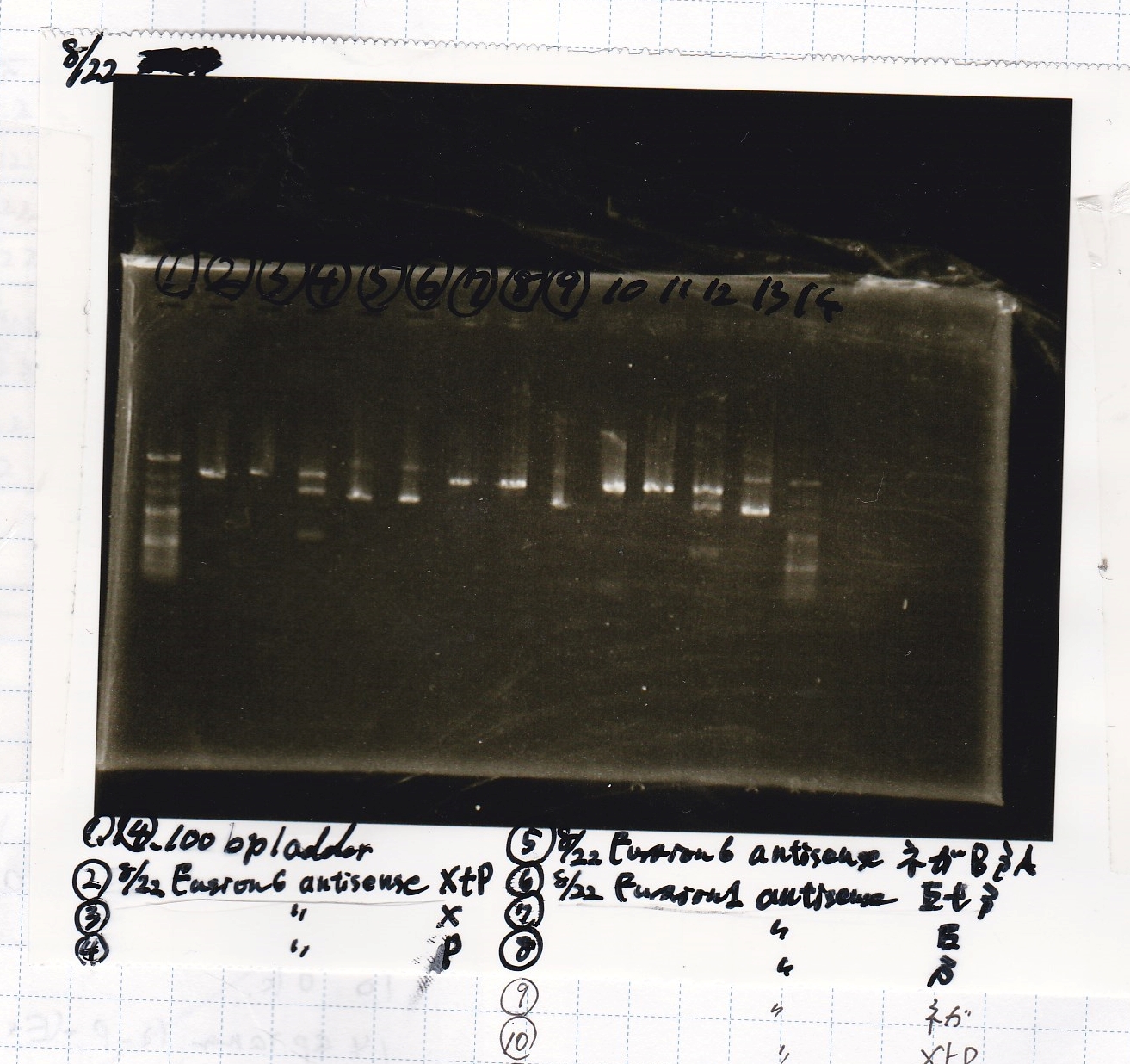
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp | -- | -- |
| 2 | 8/22 pT181 attenuator (1) | EcoRI | SpeI |
| 3 | 8/22 pT181 attenuator (1) | EcoRI | -- |
| 4 | 8/22 pT181 attenuator (1) | -- | SpeI |
| 5 | 8/22 pT181 attenuator (1) | -- | -- |
| 6 | 8/22 pT181 attenuator (1) | XbaI | PstI |
| 7 | 8/22 pT181 attenuator (1) | XbaI | -- |
| 8 | 8/22 pT181 attenuator (1) | -- | PstI |
| 9 | 8/22 pT181 attenuator (1) | -- | -- |
| 10 | 8/22 Fusion6 antisense (1) | EcoRI | SpeI |
| 11 | 8/22 Fusion6 antisense (1) | EcoRI | -- |
| 12 | 8/22 Fusion6 antisense (1) | -- | SpeI |
| 13 | 8/22 Fusion6 antisense (1) | -- | -- |
| 14 | 100bp | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp | -- | -- |
| 2 | 8/22 Fusion6 antisense (1) | XbaI | PstI |
| 3 | 8/22 Fusion6 antisense (1) | XbaI | -- |
| 4 | 8/22 Fusion6 antisense (1) | -- | PstI |
| 5 | 8/22 Fusion6 antisense (1) | -- | -- |
| 6 | 8/22 Fusion1 antisense (1) | EcoRI | SpeI |
| 7 | 8/22 Fusion1 antisense (1) | EcoRI | -- |
| 8 | 8/22 Fusion1 antisense (1) | -- | SpeI |
| 9 | 8/22 Fusion1 antisense (1) | -- | -- |
| 10 | 8/22 Fusion1 antisense (1) | XbaI | PstI |
| 11 | 8/22 Fusion1 antisense (1) | XbaI | -- |
| 12 | 8/22 Fusion1 antisense (1) | -- | PstI |
| 13 | 8/22 Fusion1 antisense (1) | -- | -- |
| 14 | 100bp | -- | -- |
 "
"