Header
Overview :
After we decided to use nanoparticles as our carrier, we had to find which kind of nanoparticles would suit our project the best. We first thought about polymers, but we needed something that could easily be degraded. So, we decided to use gelatin. It can be specifically degraded by enzymes. What's more, we found that many different kinds of gelatin nanoparticles had been made, with various loadings, such as proteins, organic molecules or DNA. We made our gelatin nanoparticles using a modified two-step desolvation method, and tried loading them with fluorescently labeled dextran and recombinant GFP. We also labeled the nanoparticles directly with a dye. What's more, all of them were biotinylated, so that they could be linked to bacteria expressing streptavidin on their surface.
What we did :
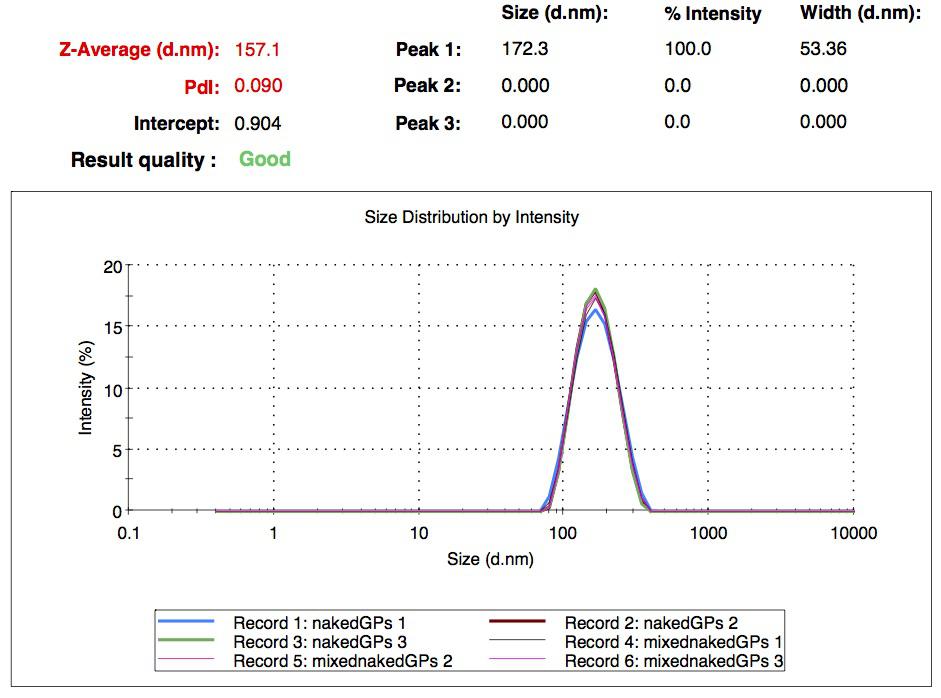
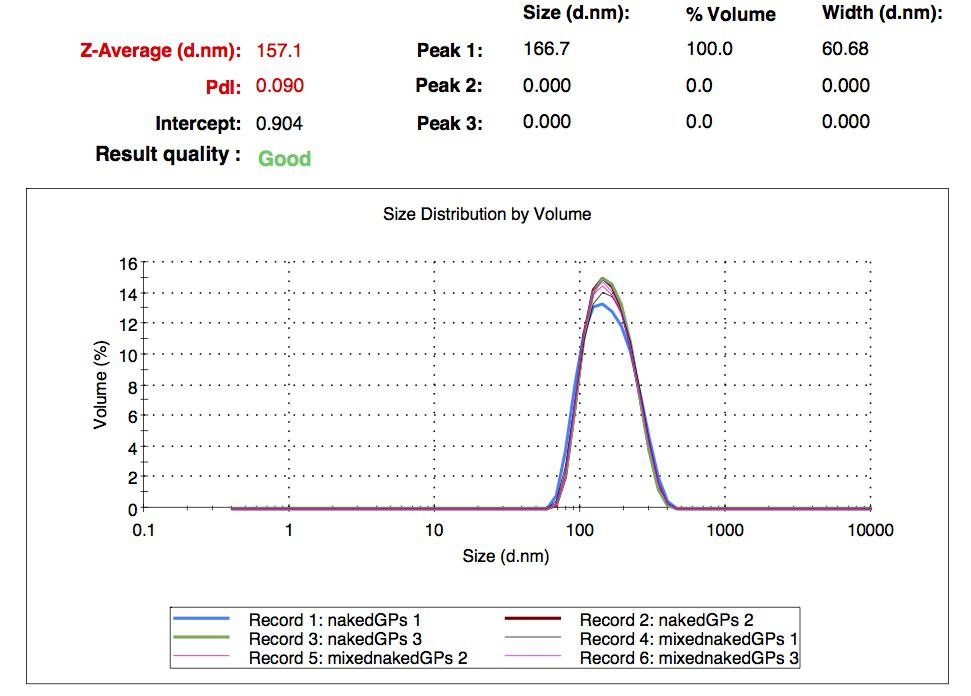
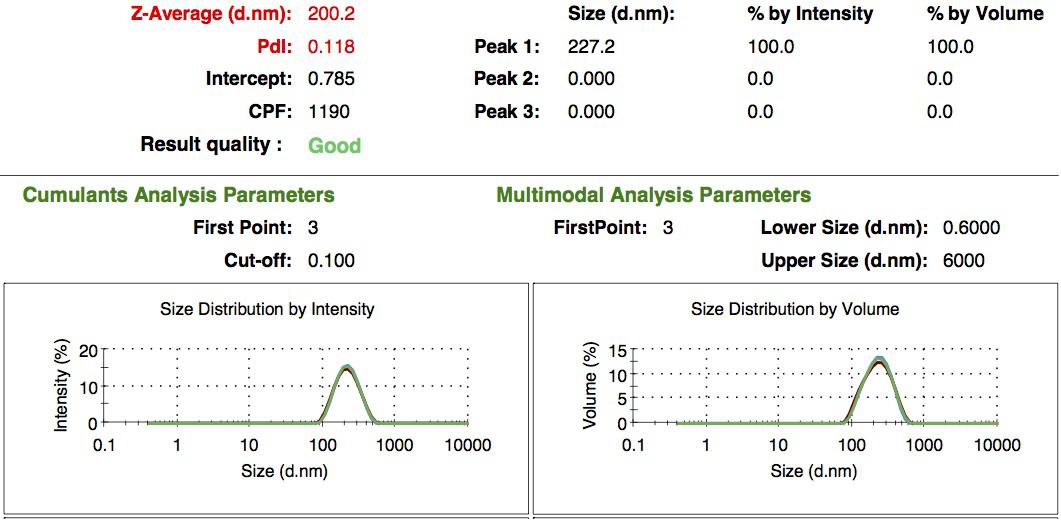
We made our gelatin nanoparticles using a modified two-step desolvation method [http://www.ncbi.nlm.nih.gov/pubmed/19534472 (Ref. 1) ]. We characterized them using dynamic light scattering (DLS). This technique allows the determination of the size distribution profile of small particles in suspension. Indeed, small particles scatter light in all direction (Rayleigh scattering), but also undergo brownian motion. Hence, the intensity of the scattered light as a function of time is representative of the size of the particles. The first particles we made with this protocol have an average diameter of about 200 nm.
</br>
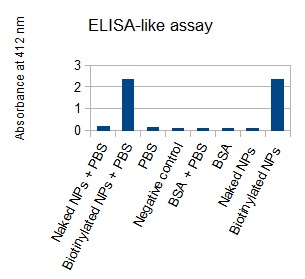
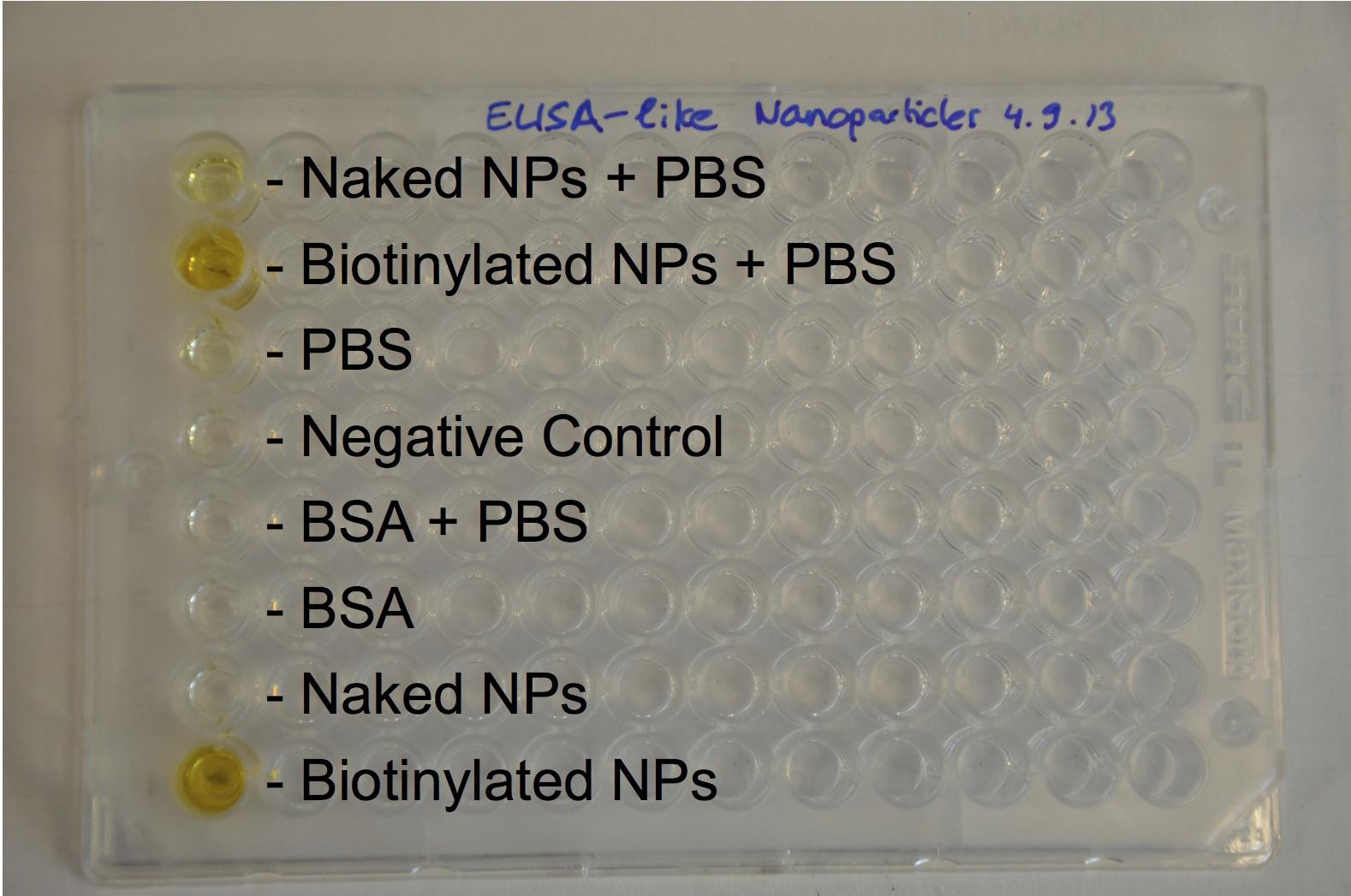
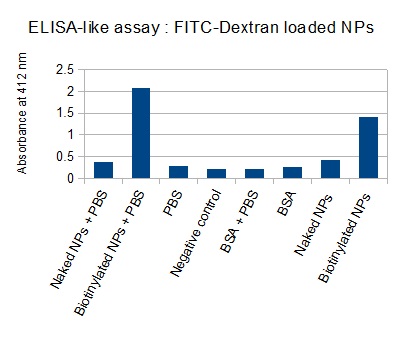
The next step was to biotinylate our nanoparticles. Biotin is a very small vitamin (244Da) that binds very strongly to avidin and streptavidin proteins. Biotinylation was made using activated biotin (Sulfo-NHS-LC-LC-Biotin), which binds primary amino groups (-NH2) and forms stable amide bonds. We used an ELISA-like assay to assess the biotinylation. It showed that biotin was indeed present on the nanoparticles.
</br>
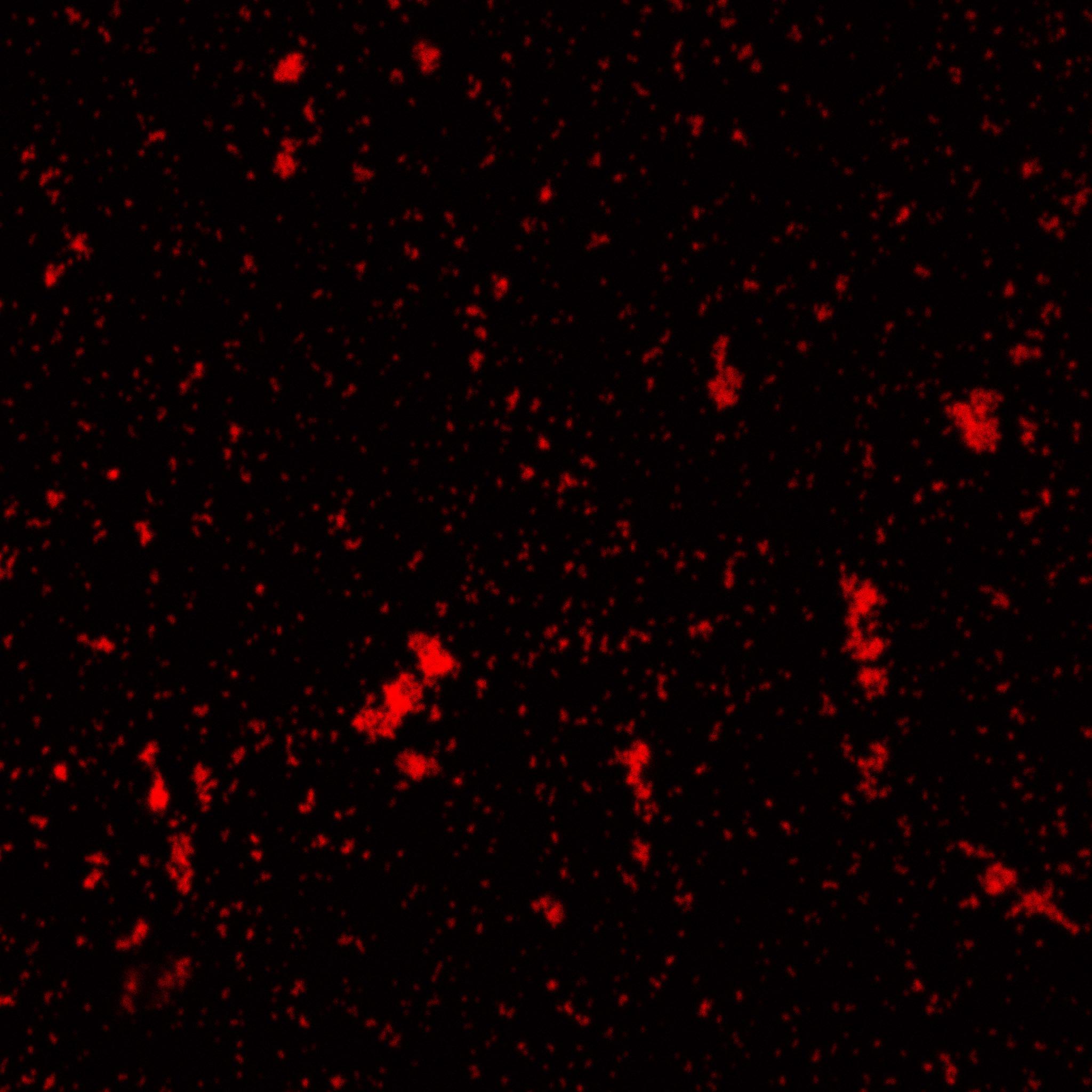
The biotinylated gelatin nanoparticles were labeled with an NHS-ester dye. This allowed visualisation of the nanoparticles with a confocal microscope.
</br>
We can see that the nanoparticles were successfully labeled. However, there were many clusters. Hence, a second image was taken (using a fluorescent microscope) after sonication.
</br>
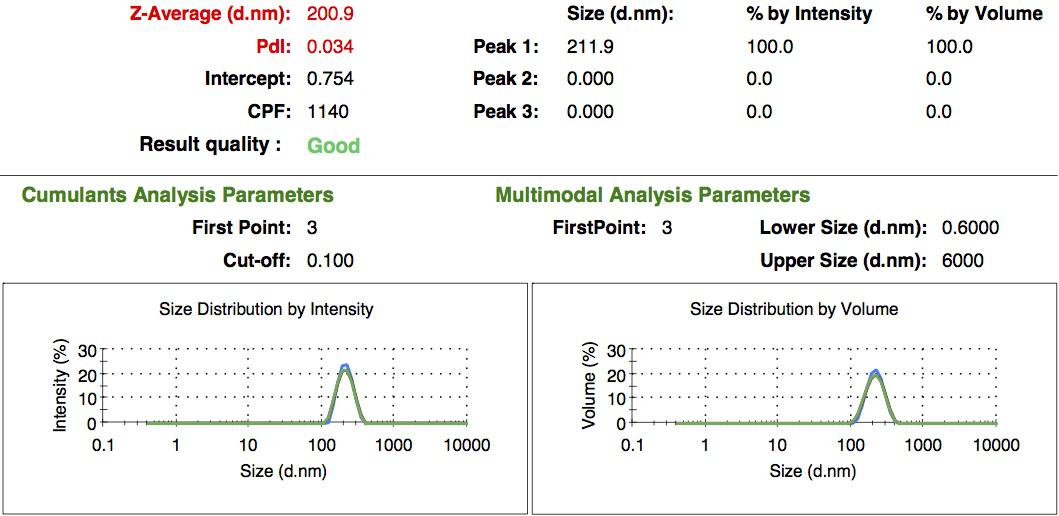
We made two new batches of gelatin nanoparticles using the same modified two-step desolvation method. This time, we loaded them with recombinant GFP and FITC-labeled dextran. The new nanoparticles have a mean diameter of about 200nm, as shown by the DLS measurements. We tested the loading with microscopy. Though the FITC-dextran was successfully introduced into the nanoparticles, it was not the case for the recombinant GFP. It is probably due to its smaller molecular weight, which allowed it to come out of the nanoparticles. What's more, the procedure implies incubation in acetone, which denatures proteins.
</br>
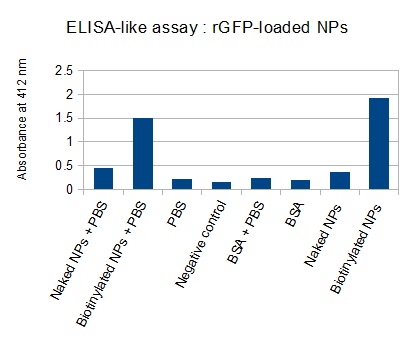
The FITC-dextran-loaded nanoparticles and the rGFP-loaded nanoparticles were biotinylated using the same activated biotin as for the previous nanoparticles. Biotinylation was successful, as shown by the ELISA-like assay.
</br>
We thought about making a third batch of nanoparticles, loading them with antibodies. However, we wanted to test first whether they could survive the low pH and the acetone. We found that the low pH (2.5) didn't denature antibodies. However, the acetone does. Thus, what could be done is to chemically attach antibodies on the nanoparticles surface, as done with NHS-ester dye (see above). Something similar has been done at the National Taiwan University [http://www.ncbi.nlm.nih.gov/pubmed/18436301 (Ref. 2)].
References :
Reference 1 : Layer-by-Layer-Coated Gelatin Nanoparticles as a Vehicle for Delivery of Natural Polyphenols.
Reference 2 : Targeting efficiency and biodistribution of biotinylated-EGF-conjugated gelatin nanoparticles administered via aerosol delivery in nude mice with lung cancer.
 "
"