Team:Edinburgh/Project/Appendix
From 2013.igem.org
Appendix
Ethanol production
Figure 1. Annotated sequence of the BBa_K173003. Underlined parts show binding sites for the MABEL primers. Sequence with red background will be deleted following blunt- ended ligation
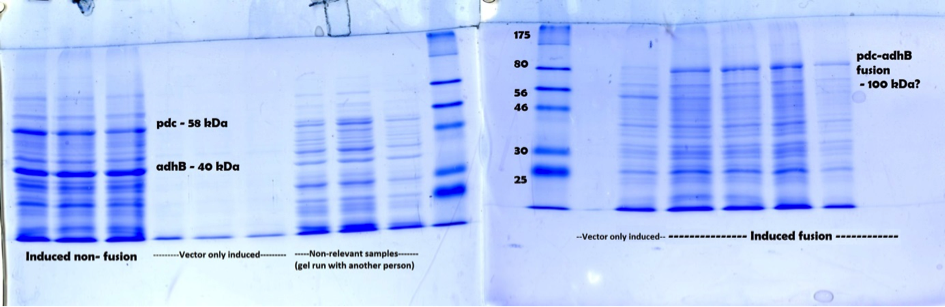
Figure 2. Full annotated SDS-PAGE result showing induced fusion, non-fusion and vector samples. For both gels a broad range protein ladder from NEB was used.
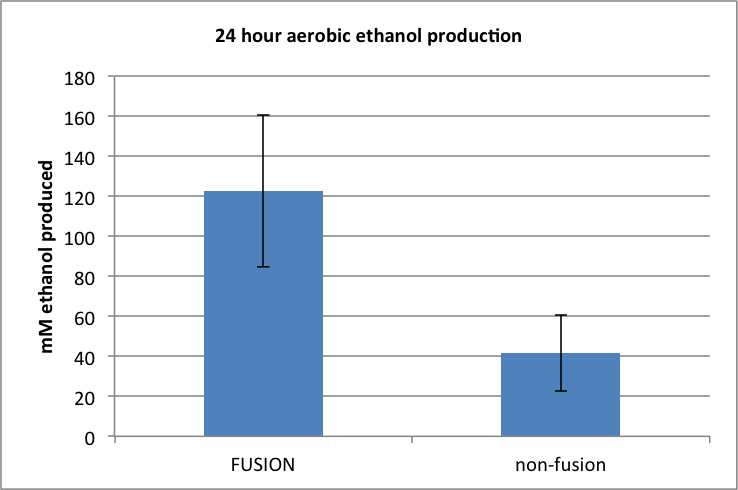
Figure 3. Amount of ethanol produced following 24h aerobic incubation.
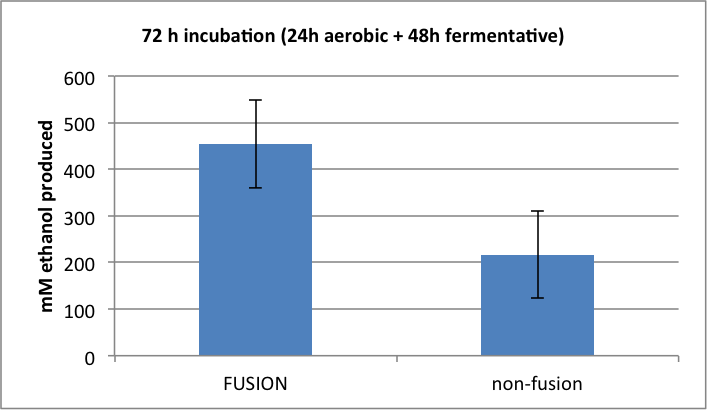
Figure 4. Amount of ethanol produced following 72h mixed incubation.
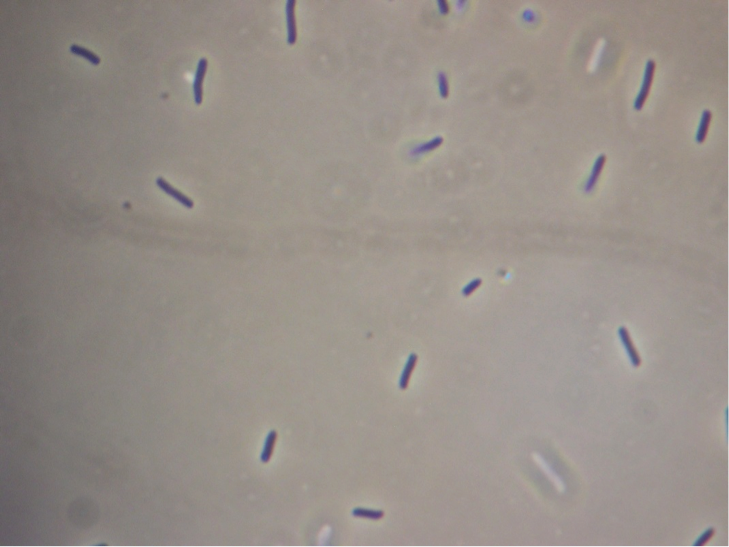
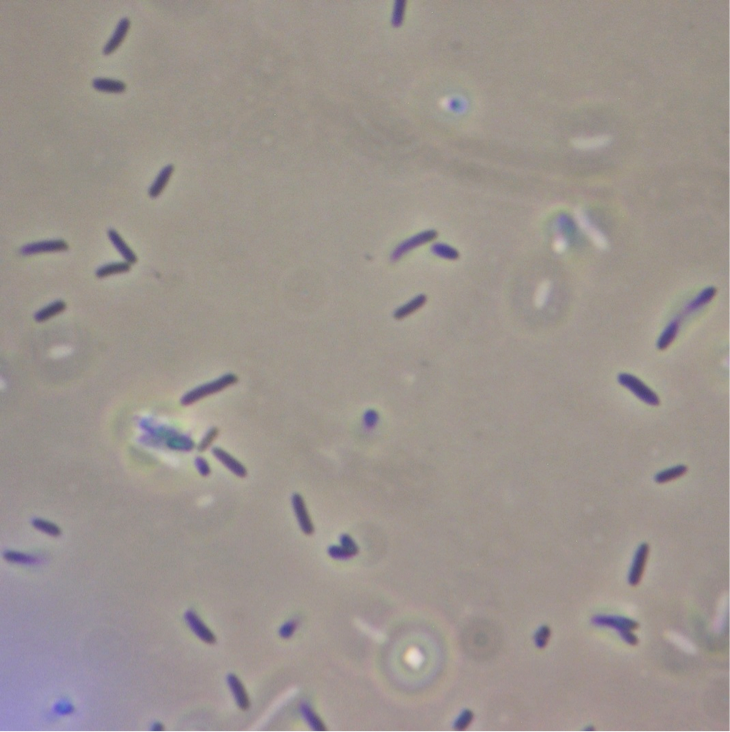
Figure 5. Full microscopic image obtained for JM109 ‘’E. coli’’ expressing fused pET cassette.
Figure 6. Full microscopic image obtained for JM109 ‘’E. coli’’ bearing fused pET cassette but not induced to express it.
Figure 7. Full microscopic image obtained for JM109 ‘’E. coli’’ expressing non-fused pET cassette.
Figure 8. Alcohol assay standard readout.
Presented below is the detailed description of MABEL based conversion of pLac to pSpac:
>BBa_J33207 (Plac + lacZ') Part-only sequence (600 bp)
ctcatgttatatcccgccgttaaccaccatcaaacaggattttcgcctgc tggggcaaaccagcgtggaccgcttgctgcaactctctcagggccaggcg gtgaagggcaatcagctgttgcccgtctcactggtgaaaagaaaaaccac cctggcgcccaatacgcaaaccgcctctccccgcgcgttggccgattcat taatgcagctggcacgacaggtttcccgactggaaagcgggcagtgagcg caacgcaattaatgtgagttagctcactcattaggcaccccaggctttac actttatgcttccggctcgtatgttgtgtgaaattgtgagcggataacaa tttcacacaggaaacagctatgaccatgattacggattcactggccgtcg ttttacaacgtcgtgactgggaaaaccctggcgttacccaacttaatcgc cttgcagcacatccccctttcgccagctggcgtaatagcgaagaggcccg caccgatcgcccttcccaacagttgcgcagcctgaatggcgaatggcgct ttgcctggtttccggcaccagaagcggtgccggaaagctggctggagtga
Underlined: promoter -35, -10, and LacI binding site
>BBa_J15503 (Pspac) Part-only sequence (284 bp)
cccagtccagactattcggcactgaaattatgggtgaagtggtcaagacc tcactaggcaccttaaaaatagcgcaccctgaagaagatttatttgaggt agcccttgcctacctagcttccaagaaagatatcctaacagcacaagagc ggaaagatgttttgttctacatccagaacaacctctgctaaaattcctga aaaattttgcaaaaagttgttgactttatctacaaggtgtggcataatgt gtggaattgtgagcggataacaattaagcttaag
Underlined: promoter start (used in vectors etc), -35, -10, and LacI binding site. Italics: additional identical bases with J32207. Capital A: +1 of mRNA.
To convert Plac to Pspac requires addition of 36 new bases upstream of the homology region. This is too much for regular PCR with standard primers but easily in range for MABEL, which would be a simpler procedure in any case. We therefore propose to design two primers which can be used to convert any J33207-controlled construct to a spac construct by MABEL.
Note that there is a further 6 bases of upstream homology separated by a single base change, A to G. Since the complementary base will be T, and G-T mismatches are relatively stable, this can also count towards homology for primer design.
Forward primer:
tatctacaaggtgtggcataatgtgtggaattgtgagcggataacaatt
(total 49 bases; underlined bases are homologous)
Reverse primer complement:
ggcaaaccagcgtggaccgc ttgc aaaaagttgttgactt
The first part of this corresponds to bases 54 to 77 of J33207. The last four of these also match the first four bases of the sequence being introduced.
Reverse primer sequence:
aagtcaacaacttttt gcaa gcggtccacgctggtttgcc
Using these primers, any circular construct containing a J33207 promoter (including almost all expression constructs made in the French lab) will be converted to a construct with a new spac promoter BioBrick instead; this will retain the LacI binding site as well as lacZ' of J32207, so if there is any expression at all in E. coli (as we might expect), blue-white selection can still be used when transferring the cassette into a vector such as pTG262.
The complete sequence of the new promoter BioBrick will be:
ctcatgttatatcccgccgttaaccaccatcaaacaggattttcgcctgc
tggggcaaaccagcgtggaccgcttgcaaaaagttgttgactttatctac
aaggtgtggcataatgtgtggaattgtgagcggataacaatttcacacag
gaaacagctatgaccatgattacggattcactggccgtcgttttacaacg
tcgtgactgggaaaaccctggcgttacccaacttaatcgccttgcagcac
atccccctttcgccagctggcgtaatagcgaagaggcccgcaccgatcgc
ccttcccaacagttgcgcagcctgaatggcgaatggcgctttgcctggtt
tccggcaccagaagcggtgccggaaagctggctggagtga
Underlined: bases which are from J15503. Italic: region of difference from J33207.
This should be given a new BioBrick code, entered into the Registry and labelled as 'spac promoter plus lacZ' alpha peptide'.

| 
| | | | 
|
| This iGEM team has been funded by the MSD Scottish Life Sciences Fund. The opinions expressed by this iGEM team are those of the team members and do not necessarily represent those of MSD | |||||
 "
"