Team:Goettingen/Team/Reporter
From 2013.igem.org
FMCommmichau (Talk | contribs) |
(→Discussion) |
||
| (72 intermediate revisions not shown) | |||
| Line 5: | Line 5: | ||
}); | }); | ||
</script> | </script> | ||
| - | + | ||
| - | + | ||
</div><!--close header--> | </div><!--close header--> | ||
<br /> | <br /> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/6/66/Goe-qNav.png" style="position: fixed;z-index: 199;bottom: 11%;right: 2.5%;_position: absolute;_top: expression(documentElement.scrollTop + documentElement.clientHeight-this.offsetHeight);cursor: pointer;opacity: 0.5;-moz-opacity: 0.5;-khtml-opacity: 0.5;-filter: alpha(opacity = 50 );" onclick="toggle(this.nextElementSibling || getnextElementSibling(this))" /> | ||
| + | <div style="position: fixed;z-index: 199;bottom: 19%;right: 2.5%;_position: absolute;_top: expression(documentElement.scrollTop + documentElement.clientHeight-this.offsetHeight);cursor: pointer;display: none;background: #fff;border: 1px solid #A3A3A3;padding: 20px; | ||
| + | border-radius: 5px;"> | ||
| + | <b>Reporter Team:</b><br /> | ||
| + | <ul> | ||
| + | <li><a href="#Introduction">Introduction</a></li> | ||
| + | <li><a href="#DarR_reporter_system_.28part_BBa_K1045017.29:">DarR reporter system</a></li> | ||
| + | <li><a href="#Riboswitch_reporter_system_.28part_BBa_K1045002.29:">Riboswitch system</a></li> | ||
| + | <li><a href="#Results">Results</a></li> | ||
| + | <li><a href="#Discussion">Discussion</a></li> | ||
| + | </ul> | ||
| + | </div> | ||
| + | |||
| - | |||
| - | |||
<div id="body"> | <div id="body"> | ||
<div class="f-box"> | <div class="f-box"> | ||
| Line 17: | Line 27: | ||
<div id="nav"> | <div id="nav"> | ||
</html> | </html> | ||
| - | + | {{:Team:Goettingen/templates/navProject}} | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
=== === | === === | ||
| Line 35: | Line 37: | ||
<div id="col-right" class="bl"> | <div id="col-right" class="bl"> | ||
<br /> | <br /> | ||
| - | + | <img src="https://static.igem.org/mediawiki/2013/5/5a/Goe-bingyaoZhu.jpg" style="display:inline;height:150px" /> | |
| + | <img src="https://static.igem.org/mediawiki/2013/5/5c/Goe-dominikBecker.JPG" style="display:inline;height:150px" /> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/7/78/Goe-katrinTreffon.jpg" style="display:inline;height:150px" /> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/e/e5/Goe-ninaHeckmann.jpg" style="display:inline;height:150px" /> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/f/f1/Goe-jonathanRosenberg.jpg" style="display:inline;height:150px" /> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/5/56/Goe-greenColi-reporter.png" style="display:inline;height:150px" /> | ||
</html> | </html> | ||
===Reporter Team=== | ===Reporter Team=== | ||
| + | ==Introduction== | ||
| + | In order to facilitate the development of new antibiotics, we want to build an “Antibiotic Detector” that can identify substances, affecting homeostasis of cyclic-di-AMP (c-di-AMP). c-di-AMP is a signal nucleotide that is essential for viability of many Gram-positive bacteria. It is not found in humans as well as in Gram-negative bacteria, including <i>E. coli</i>. For more information see [[Team:Goettingen/Project/OurProject|Our Project overview]]. | ||
| + | |||
| + | For constructing a c-di-AMP detector, we need elements that are capable of sensing c-di-AMP! Moreover, in order to visualize the output of the sensors, we need a reporter. | ||
| + | |||
| + | ==DarR reporter system ([http://parts.igem.org/Part:BBa_K1045017 part BBa_K1045017]):== | ||
| + | |||
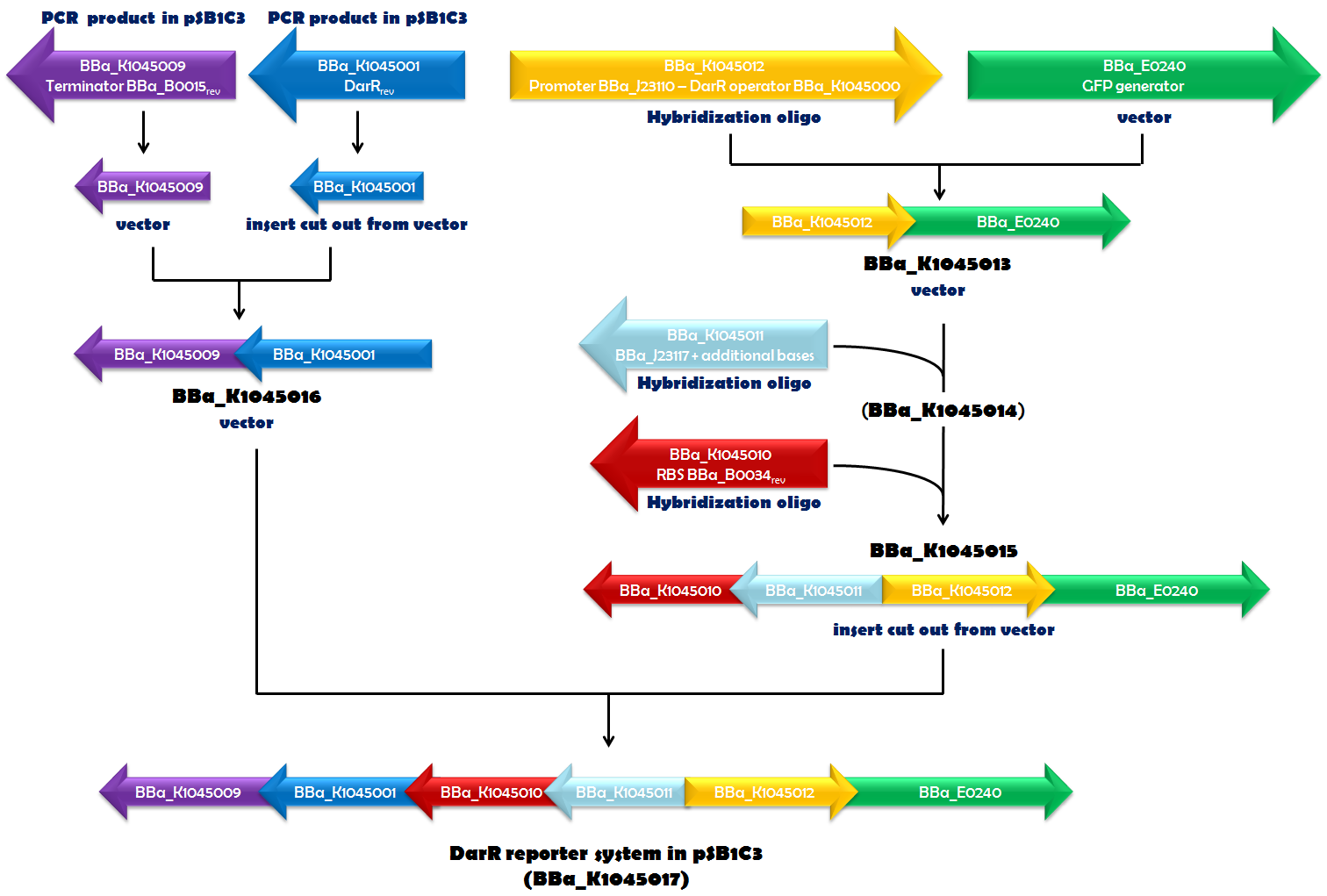
| + | Recently, in ''Mycobacterium smegmatis'', the transcriptional repressor DarR was identified and shown to respond to c-di-AMP. It can bind to a specific DNA sequence, called the DarR operator. DNA-binding activity of DarR is strongly enhanced by c-di-AMP. Since DarR is a c-di-AMP sensor, we intended to use it for our reporter system. We cloned the DNA sequence, encoding DarR into pSB1C3 ([http://parts.igem.org/Part:BBa_K1045001 BBa_K1045001]). In addition to this, we constructed the DarR operator as a Biobrick ([http://parts.igem.org/Part:BBa_K1045000 BBa_K1045000]). We placed the DarR operator between a strong promoter ([http://parts.igem.org/Part:BBa_J23110 BBa_J23110]) and the GFP generator [http://parts.igem.org/Part:BBa_E0240 BBa_E0240]. In the very same plasmid but oriented in the opposite direction, we assembled a <i>darR</i> expression unit. Synthesis of DarR is driven by a weak promoter based on [http://parts.igem.org/Part:BBa_J23117 BBa_J23117] and terminated by [http://parts.igem.org/Part:BBa_K1045009 BBa_K1045009]. This terminator is based on [http://parts.igem.org/Part:BBa_B0015 BBa_B0015], which is part of [http://parts.igem.org/Part:BBa_E0240 BBa_E0240], as well, and should ensure that transcription of <i>darR</i> and <i>gfp</i> does not influence each other. The ribosome-binding site (RBS) [http://parts.igem.org/Part:BBa_K1045010 BBa_K1045010] (derived from [http://parts.igem.org/Part:BBa_B0034 BBa_B0034]) was used as an RBS for translation of the <i>darR</i> mRNA. Assembly of all those parts (Fig. 1.1) required a complex cloning process which finally resulted in part [http://parts.igem.org/Part:BBa_K1045017 BBa_K1045017], the DarR reporter system (Fig. 1.2). ''E. coli'' was then transformed with this construct to obtain an ''in vivo'' screening system that interfere with c-di-AMP-binding to a c-di-AMP target. | ||
| + | |||
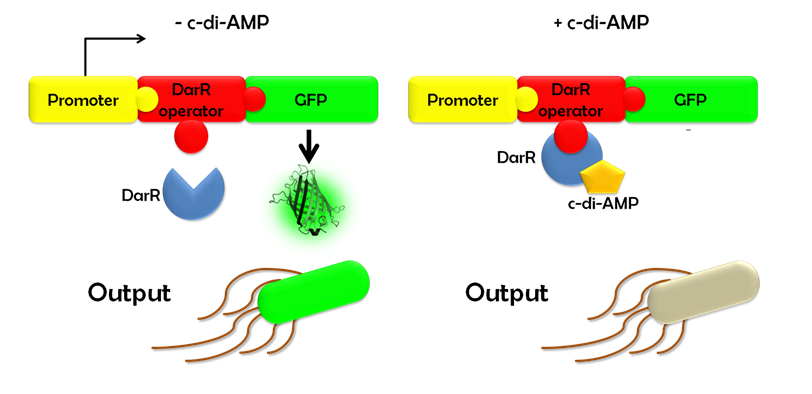
| + | Ideally, this c-di-AMP-sensing system works in the following way (Fig. 1.3): Without c-di-AMP, DarR is not bound to the DarR operator, so GFP is expressed resulting in green fluorescing cells. With c-di-AMP, DarR can bind to its binding sequence, repressing <i>gfp</i> transcription, leading to non-fluorescent cells. In the same way, the system might react to compounds similar to c-di-AMP. It can therefore be used to screen for compounds similar to c-di-AMP. It can be used in a high-throughput scale, as well. This way, the DarR reporter system will make it possible to find inhibitors and competitors which might be used as antibiotics. | ||
| + | |||
| + | <html><a href="https://static.igem.org/mediawiki/2013/e/ea/Goe-rt-cloningSteps.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/e/ea/Goe-rt-cloningSteps.png" width="750"/></a></html> | ||
| + | |||
| + | ''Fig. 1.1. The DarR reporter system was assembled step by step in pSB1C3. If a certain biobrick of a specific cloning step was used as a vector, insert or derived from hybridization oligos or PCR is indicated in blue. The part numbers of the intermediates are given in black. The orientation of the arrows corresponds to the orientation of the element in the vector.'' | ||
| + | |||
| + | <html><a href="https://static.igem.org/mediawiki/2013/6/6c/Goe-rt-assemblingStrategy.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/6/6c/Goe-rt-assemblingStrategy.png" width="750" /></a></html> | ||
| + | |||
| + | ''Figure 1.2: The final DarR reporter system consisted of two expression units in diverging orientation.'' | ||
| + | |||
| + | <html><a href="https://static.igem.org/mediawiki/2013/f/f6/Goe-rt-DarRsystem.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/f/f6/Goe-rt-DarRsystem.png" width="750"></a></html> | ||
| + | |||
| + | |||
| + | ''Figure 1.3: Without c-di-AMP, <i>E. coli</i> cells expressing the DarR reporter system will emit a green fluorescence signal. With c-di-AMP the will not fluoresce.'' | ||
| + | |||
<html> | <html> | ||
| - | <p> | + | <p><b>Reference</b></p> |
| + | <p>Zhang <i>et al.</i> (2013) DarR, a TetR-like transcriptional factor, Is a cyclic di-AMP-responsive repressor in <i>Mycobacterium smegmatis. J Biol Chem</i> 288:3085–3096.</p> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<br /> | <br /> | ||
</html> | </html> | ||
| + | <br /><br /> | ||
| + | |||
| + | ==Riboswitch reporter system ([http://parts.igem.org/Part:BBa_K1045002 part BBa_K1045002]):== | ||
| + | The ''ydaO'' gene from ''B. subtilis'' is regulated by c-di-AMP. This control is mediated by a c-di-AMP-binding riboswitch, according to data of a publication that appeared this month (Nelson ''et al.'', 2013). Structurally, a riboswitch is a RNA-sequence. Functionally, it is a simple switch. It has two mutually exclusive states: ON and OFF. Depending on the circumstances, it can only be in one of the states. The ''<i>ydaO</i>''-riboswitch is dependent on c-di-AMP; whenever it is present, the riboswitch will be turned OFF, meaning that the gene controlled by it cannot be read. | ||
| + | |||
| + | The <i>ydaO</i> riboswitch was, in addition to DarR, a perfect fit as a c-di-AMP sensor for our antibiotic detector. Hence, we constructed an alternative reporter system. | ||
| + | |||
| + | Since it was unclear where the exact beginning and ending of the ''ydaO'' promoter, the ''ydaO'' riboswitch and the ''ydaO'' RBS were, we created four different constructs (Fig. 2.; [http://parts.igem.org/Part:BBa_K1045004 BBa_K1045004], [http://parts.igem.org/Part:BBa_K1045005 BBa_K1045005], [http://parts.igem.org/Part:BBa_K1045006 BBa_K1045006], [http://parts.igem.org/Part:BBa_K1045007 BBa_K1045007]). The sequence of [http://parts.igem.org/Part:BBa_K1045004 BBa_K1045004] was cloned in front of the reporter gene CFP ([http://parts.igem.org/Part:BBa_E0020 BBa_E0020]). For the termination of transcription, we exploited the terminator on pSB1C3. In principle, the riboswitch reporter system might respond in exactly the same way to c-di-AMP, as the DarR reporter system does: Without c-di-AMP, <i>E. coli</i> cells harboring [http://parts.igem.org/Part:BBa_K1045002 BBa_K1045002] will fluoresce blue. In contrast, when c-di-AMP is present, the ''ydaO'' riboswitch will terminate transcription of ''cfp'' leading to non-fluorescent cells (Fig. 2.2). This way, we can scan substances which can act on the riboswitch as c-di-AMP does. This might lead to the identification of competitors or inhibitors for future antibiotics, as well. | ||
| + | |||
| + | <html><a href="https://static.igem.org/mediawiki/2013/4/44/Goe-rt-riboswitchsystem.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/4/44/Goe-rt-riboswitchsystem.png" width="750"></a></html> | ||
| + | |||
| + | ''Figure 2.1. The riboswitch reporter system is composed of the ydaO riboswitch and cfp as a reporter gene.'' | ||
| + | |||
| + | <html><a href="https://static.igem.org/mediawiki/2013/c/cd/Goe-rt-riboRsystem.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/c/cd/Goe-rt-riboRsystem.png" width="750" /></a></html> | ||
| + | |||
| + | ''Figure 2.2. In theory, ''E. coli'' cells transformed with the riboswitch reporter system will fluoresce blue when c-di-AMP is absent. When c-di-AMP is present, however, the ''ydaO'' riboswitch will prevent expression of CFP.'' | ||
| + | |||
| + | <p><b>References</b></p> | ||
| + | <p>Watson, P. Y. & Fedor, M. J. (2012) The ''ydaO'' motif is an ATP-sensing riboswitch in ''Bacillus subtilis'', ''Nat Chem Biol'' 8: 963-96.</p> | ||
| + | <p>Nelson ''et al.'' (2013) Riboswitches in eubacteria sense the second messenger c-di-AMP. ''Nat Chem Biol'' 8: 963-96. doi: 10.1038/nchembio.1363.</p> | ||
| + | |||
| + | |||
| + | ===Results=== | ||
| + | |||
| + | ==The DarR Reporter System== | ||
| + | |||
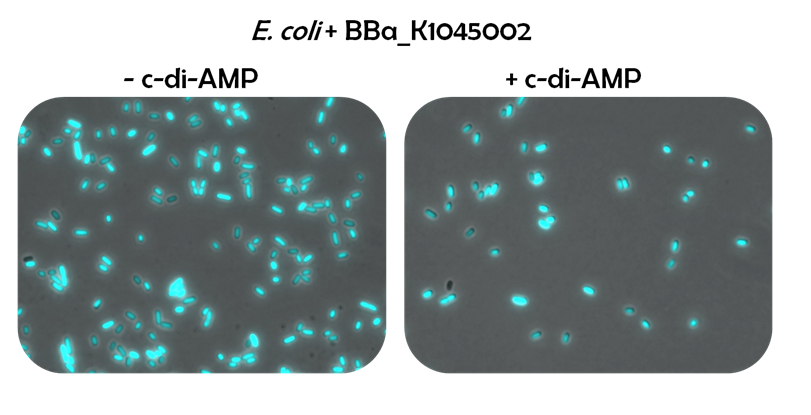
| + | To test the DarR reporter system, ''E. coli'' cells were transformed with the DarR reporter system ([http://parts.igem.org/Part:BBa_K1045017 BBa_K1045017]) (Fig. 3, right image) or with a control plasmid containing the GFP expression unit only ([http://parts.igem.org/Part:BBa_K1045013 BBa_K1045013]) (Fig. 3, left image). Both strains were grown without c-di-AMP and subjected to fluorescence microscopy. While the control strain showed green fluorescing cells, the cells of the DarR reporter system strain barely fluoresced, indicating that DarR acts as a strong repressor in ''E. coli''. | ||
| + | |||
| + | <html><a href="https://static.igem.org/mediawiki/2013/a/a4/Geo-rt-re-darr.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/a/a4/Geo-rt-re-darr.png" width="750" /></a></html> | ||
| + | |||
| + | ''Figure 3: To test the DarR reporter system, fluorescence microscopy of E. coli transformed with a control plasmid (left) and E.coli cells harboring the DarR reporter system (right) was done. Both images are merges of GFP channel and bright field.'' | ||
| + | |||
| + | ==The Riboswitch Reporter System== | ||
| + | |||
| + | For testing the riboswitch reporter system, ''E.coli'' was transformed with [http://parts.igem.org/Part:BBa_K1045002 BBa_K1045002] and grown either with c-di-AMP or without. The cells were analyzed by fluorescence microscopy. A strong fluorescence for both strains could be observed, indicating that the reporter construct is highly expressed from the ''B. subtilis'' promoter. However, an influence of c-di-AMP was not seen. | ||
| + | |||
| + | <html><a href="https://static.igem.org/mediawiki/2013/f/fa/Geo-rt-re-ribo.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/f/fa/Geo-rt-re-ribo.png" width="750" /></a></html> | ||
| + | |||
| + | ''Figure 4: E. coli cells containing the riboswitch reporter system were grown without (left) or with (right) c-di-AMP and studied under the fluorescence microscope.'' | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ===Discussion=== | ||
| + | Since we are in urgent need for novel antibiotics, we constructed two different c-di-AMP reporter systems which would allow the screening for compounds affecting c-di-AMP biofunction. One system was based on the c-di-AMP-binding transcriptional repressor DarR. In our system, DarR controlled the expression of the reporter gene GFP. ''E. coli'' cells transformed with a vector harboring the DarR reporter system exhibited no green fluorescence compared to cells transformed with a control plasmid lacking the DarR expression unit. This data indicated that DarR seems to be expressed and active as a repressor in ''E. coli''. Even in the absence of c-di-AMP, DarR was likely to bind the DarR operator strongly and repressed GFP expression almost completely. Under these conditions, an intermediate GFP expression level would be more suitable for our purpose to screen for novel antibiotics. Such an intermediate expression level of GFP in the absence of c-di-AMP might be reached in two ways: For instance, one could try a promoter for DarR even weaker than the current one. A more successful approach might be directed mutagenesis of the DarR operator to reduce the binding affinity of DarR to this DNA sequence. | ||
| + | |||
| + | Regarding the alternative reporter system involving the c-di-AMP-responsive ''ydaO'' riboswitch, optimization is needed, as well. The results obtained from fluorescence microscopy showed that the riboswitch reporter construct is expressed in ''E. coli'' though the native Bacillus promoter and Bacillus RBS were employed. Hence, ''E. coli'' seems to be able to use these elements. The strong CFP fluorescence of the ''E. coli'' cells even suggests a strong promoter activity. The high transcript levels that might be caused by this strong promoter could account for the observed ineffectiveness of exogenously applied c-di-AMP: There might be more riboswitch than c-di-AMP to bind to. Consequently, one could try to increase the c-di-AMP amounts. Alternatively, one could fuse the riboswitch biobrick [http://parts.igem.org/Part:BBa_K1045005 BBa_K1045005] to a weaker promoter to reduce the mRNA levels. Another possibility might be to switch to low-copy plasmids for expression of the reporter system. If despite reduced transcript levels, c-di-AMP has no effect on the riboswitch reporter system, ''E. coli'' might be unable to take up c-di-AMP. Yet, this would not put an end to the construction of a screening system for antibiotics targeting c-di-AMP. It has been reported, that B. subtilis is able to take up c-di-AMP (Oppenheimer-Shaaman ''et al.'', 2011). Thus, one could put more effort in unraveling the ways by which ''B. subtilis'' and other bacteria take up c-di-AMP. This knowledge might allow the engineering of ''E. coli'' regarding c-di-AMP uptake. In parallel to this time-consuming approach, one could try to synthesize c-di-AMP ''in vivo'' by expressing diadenylate cyclases in ''E. coli'' cells containing an optimized c-di-AMP reporter system. Using DACs of different activity, the output of the reporter system could be characterized. Finally, having a well characterized c-di-AMP reporter system combined with a DAC, it is possible to screen ''in vivo'' for antibiotics interfering not only with the function of the essential signaling nucleotide c-di-AMP, but also for its essential biosynthesis enzyme, the DAC. | ||
| + | |||
| + | '''References:''' | ||
| + | |||
| + | Yaara Oppenheimer-Shaanan, Ezequiel Wexselblatt, Jehoshua Katzhendler, Eylon Yavin & Sigal Ben-Yehuda (2011) “c-di-AMP reports DNA integrity during sporulation in ''Bacillus subtilis''”, EMBO reports Vol. 12, No. 6, pp. 594-601 | ||
=== === | === === | ||
<html> | <html> | ||
| - | <a href="https://2013.igem.org/Team:Goettingen/Project | + | <a href="https://2013.igem.org/Team:Goettingen/Project" class="moreinfo fl"><b>Previous</b></a> |
<a href="/Team:Goettingen/Team/Array" class="moreinfo fr"><b>Next</b></a> | <a href="/Team:Goettingen/Team/Array" class="moreinfo fr"><b>Next</b></a> | ||
<br /><br /> | <br /><br /> | ||
| - | |||
</div><!--close col-right--> | </div><!--close col-right--> | ||
| + | <br /> | ||
</div> | </div> | ||
| - | |||
| - | |||
Latest revision as of 09:30, 28 October 2013
 "
"