Team:Heidelberg/Templates/Del week15 L
From 2013.igem.org
Contents |
07-08-2013
Results Sequencing
Send the following samples (amplified fragments of D. acidovorans DSM-39 and backbone pSB4K5 from the partsregistry) to GATC for sequencing:
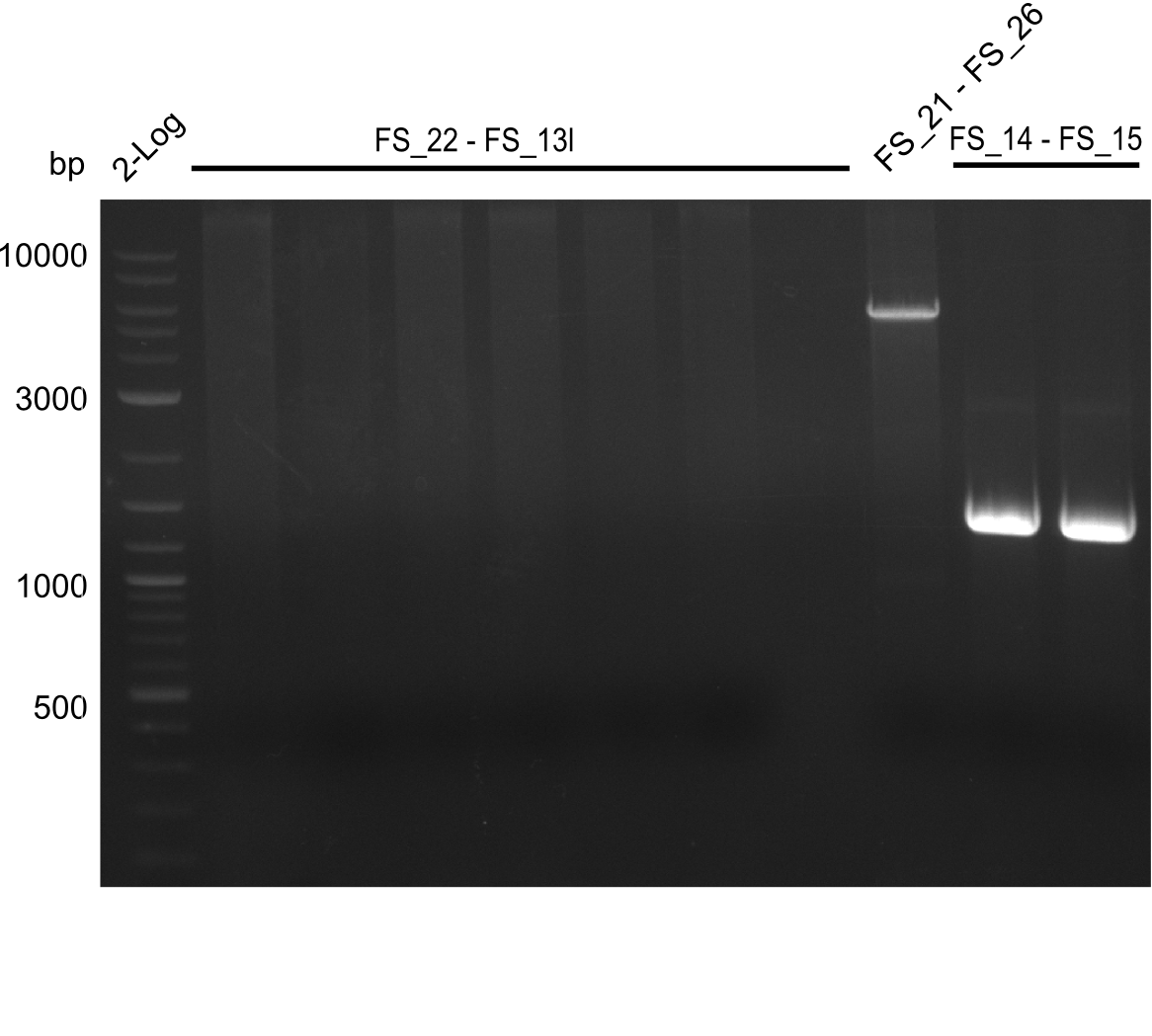
- DelL (FS_14 to FS_15) with Primer FS_14
- DelL (FS_14 to FS_15) with Primer FS_15
L-FS 14.txt
Sequencing of DelL(FS_14 to FS_15) with Primer FS_14 |
L-FS 15.txt
Sequencing of DelL(FS_14 to FS_15) with Primer FS_15 |
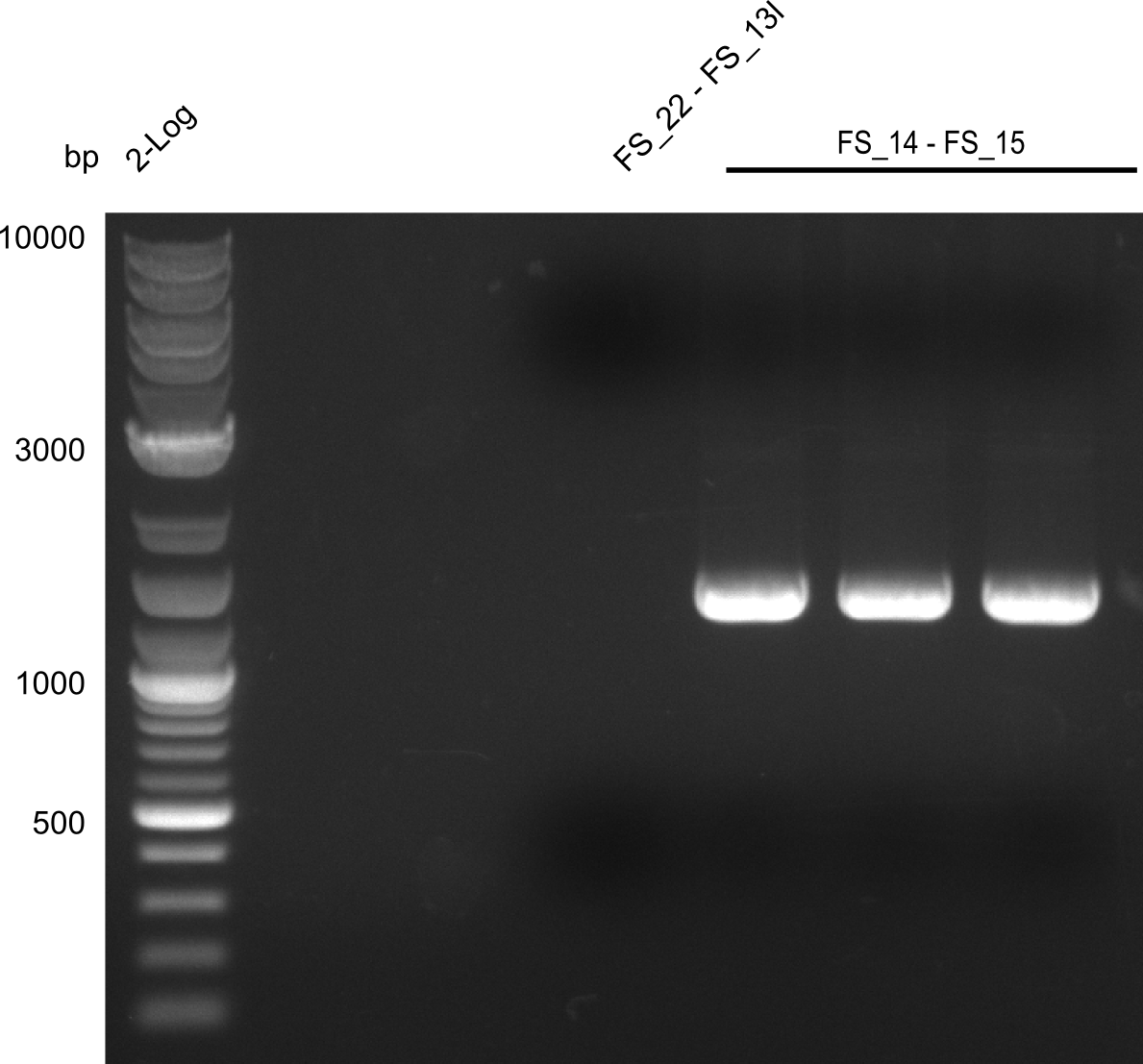
Amplification of DelL (FS_14 to FS_15; 1.4 kb)
- Reaction
| what | µl |
|---|---|
| D. acidovorans SPH-1 | 1 |
| FS_14: (1/10) | 2.5 |
| FS_15: (1/10) | 2.5 |
| Phusion Master Mix | 25 |
| DMSO | 2.5 |
| dd H2O | 16.5 |
- Conditions
| Biorad MyCycler | ||
|---|---|---|
| Cycles-PCR | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 66 ↓ 0.5 | 5 | |
| 72 | 42 | |
| 18 | 98 | 1 |
| 63 | 5 | |
| 72 | 42 | |
| 1 | 72 | 12 min |
| 1 | 4 | inf |
Results:
- Amplification worked very well, we had bright bands on the right height.
- Bands were cut out and DNA purified using QIAquick Gel Extration Kit.
08-08-2013
Concentration measurement of DelL
| Fragment | Primer | Date PCR | Concentration |
|---|---|---|---|
| DelL | FS14-FS15 | 07-08-2013 | 51 ng/µl |
09-08-2013
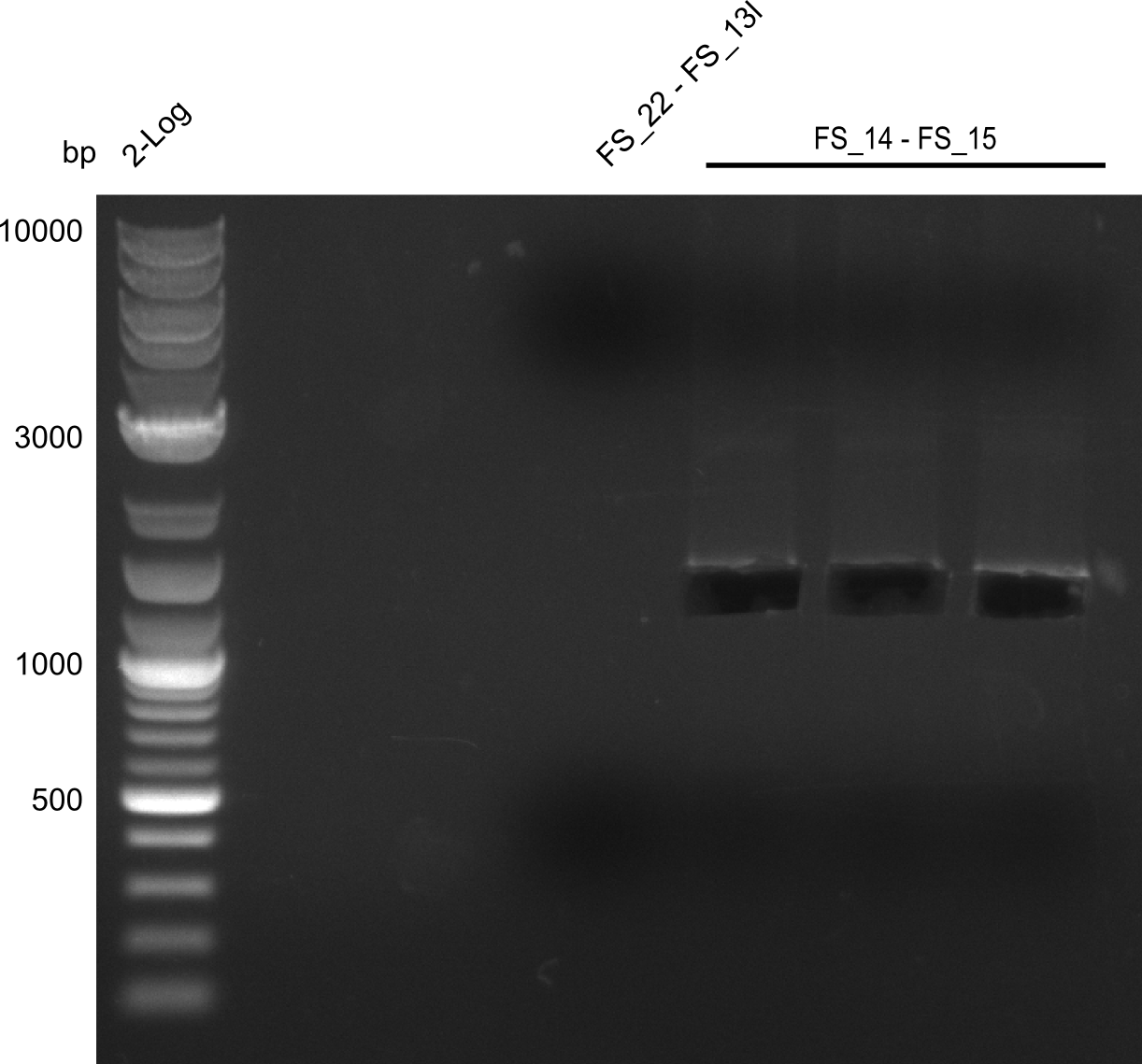
Amplification of DelL (FS_14 to FS_15; 1.4 kb)
- Reaction
| what | µl |
|---|---|
| D. acidovorans | 1 |
| FS_14: (1/10) | 2.5 |
| FS_15: (1/10) | 2.5 |
| Phusion Master Mix | 25 |
| DMSO | 2.5 |
| dd H2O | 16.5 |
- Conditions
| Biorad MyCycler* | ||
|---|---|---|
| Cycles-PCR | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 66 ↓ 0.5 | 5 | |
| 72 | 42 | |
| 18 | 98 | 1 |
| 63 | 5 | |
| 72 | 42 | |
| 1 | 72 | 12 min |
| 1 | 10 | inf |
Results:
- Amplification worked very well, we had bright bands on the right height.
- Bands were cut out and DNA purified using QIAquick Gel Extration Kit.
 "
"