Team:Heidelberg/Templates/Del week16 pSB4K5
From 2013.igem.org
(Difference between revisions)
Charly (Talk | contribs)
(Created page with "==15-08-2013== ===Amplification from FS_01 to FS_16; 4.2 kb=== [[File:Heidelberg_20130814 2log 4xDelFGstandard 2log 4xpSB4K5standard.png|150px|thumb| Amplification of DelFG and ...")
Newer edit →
(Created page with "==15-08-2013== ===Amplification from FS_01 to FS_16; 4.2 kb=== [[File:Heidelberg_20130814 2log 4xDelFGstandard 2log 4xpSB4K5standard.png|150px|thumb| Amplification of DelFG and ...")
Newer edit →
Revision as of 01:25, 30 September 2013
Contents |
15-08-2013
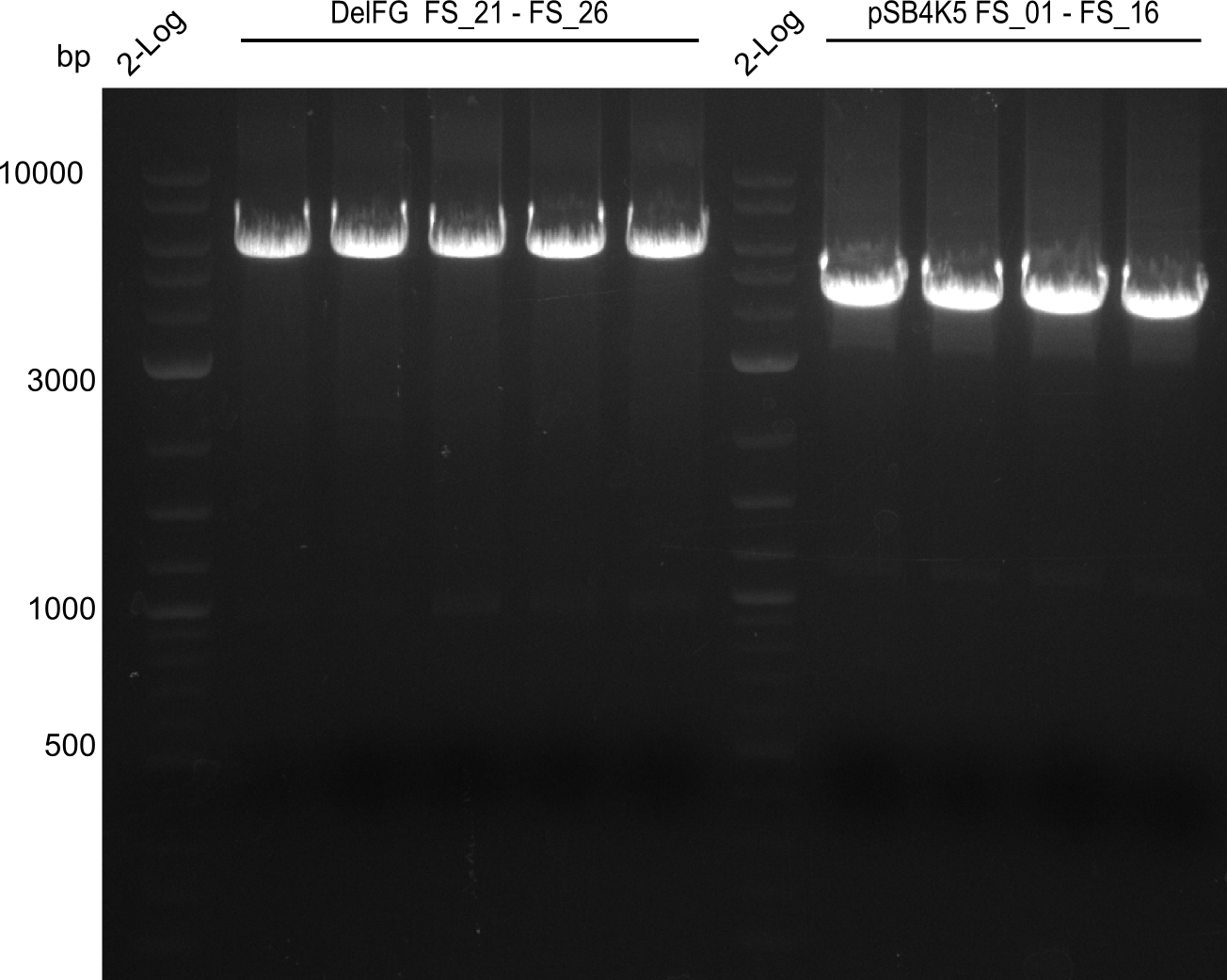
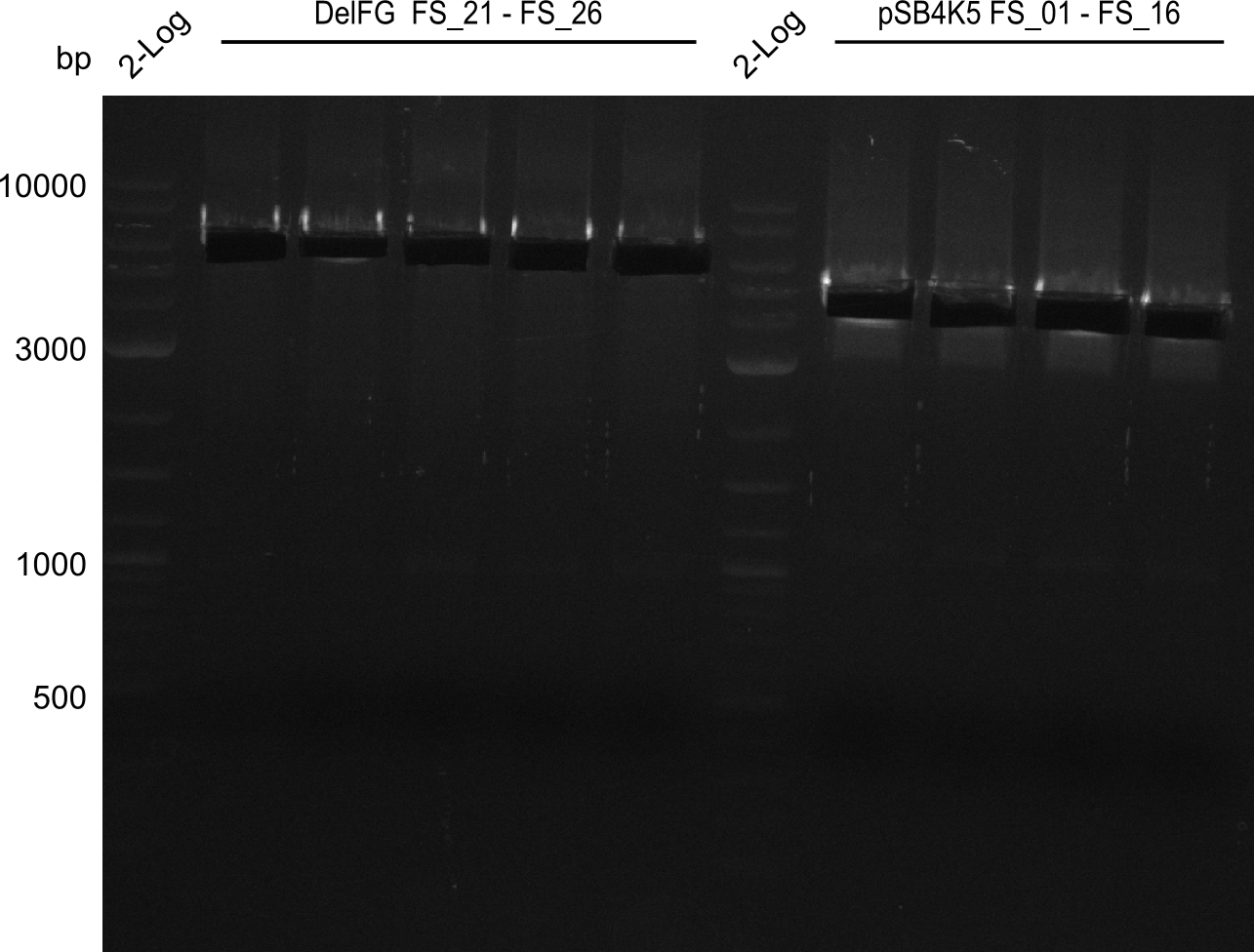
Amplification from FS_01 to FS_16; 4.2 kb
- Reaction
(2x50µl)
| what | µl |
|---|---|
| Template pSB4K5 | 1 |
| FS_01: (1/10) | 2.5 |
| FS_16: (1/10) | 2.5 |
| Phusion Master Mix | 25 |
| DMSO | 2.5 |
| dd H2O | 16.5 |
- Conditions
| Biorad T100 | ||
|---|---|---|
| Cycles-PCR | temperature [°C] | Time [s] |
| 1 | 98 | 5 |
| 12 | 98 | 1 |
| 62 | 5 | |
| 72 | 1:30 min | |
| 1 | 72 | 5 min |
| 1 | 8 | inf |
Results:
- Amplification worked very well, we had bright bands on the right height.
- Bands were cut out and DNA purified using QIAquick Gel Extration Kit.
Restriction digest of pSB4K5 (FS_01 to FS_16; 4.2 kb; 15-08-2013) with DpnI
Incubation at 37°C for about 6 hours
| what | µl |
|---|---|
| FS_16 to FS_01 (15-08-2013) | 30 |
| DpnI | 2 |
| CutSmart Buffer | 4 |
| dd H2O | 4 |
Results:
- Bands were cut out and DNA purified using QIAquick Gel Extration Kit.
16-08-2013
Concentration measurement
The concentration of the gel purified, and DpnI digested fragment was measured using a NanoDrop Instrument.
| Fragment | Primer | Date PCR | Concentration |
|---|---|---|---|
| pSB4K5 DpnI digested | FS01-FS16 | 15-08-2013 | 30 ng/µl |
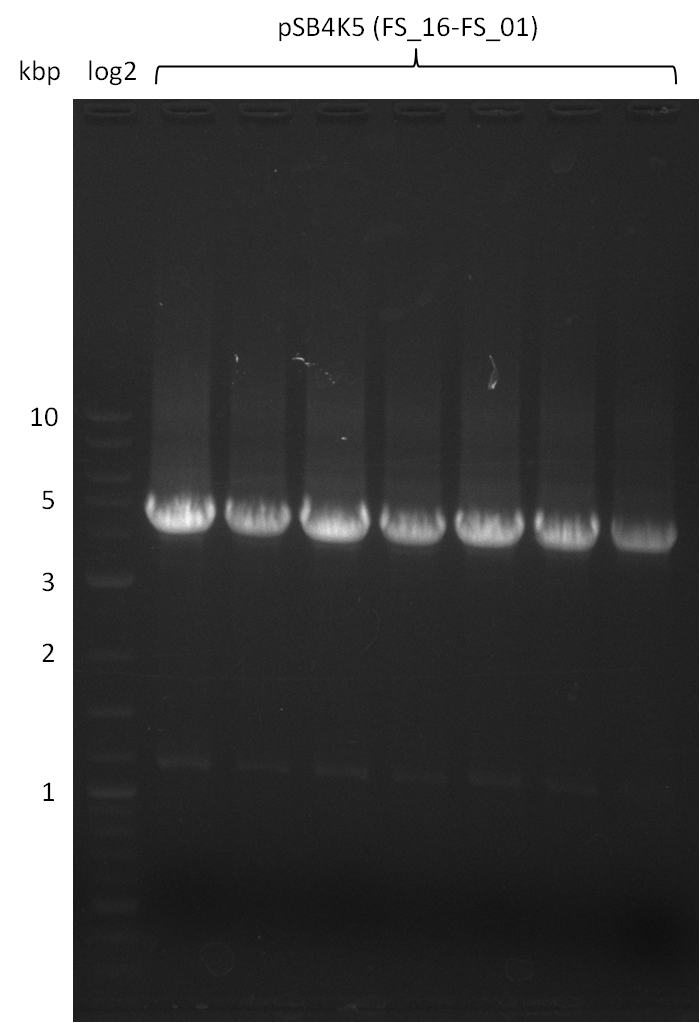
Amplification from FS_01 to FS_16; 4.2 kb
- Reaction
(3x50µl)
| what | µl |
|---|---|
| Template pSB4K5 | 1 |
| FS_01: (1/10) | 2.5 |
| FS_16: (1/10) | 2.5 |
| Phusion Master Mix | 25 |
| DMSO | 2.5 |
| dd H2O | 16.5 |
- Conditions
| Biorad T100 | ||
|---|---|---|
| Cycles-PCR | temperature [°C] | Time [s] |
| 1 | 98 | 5 |
| 12 | 98 | 1 |
| 62 | 5 | |
| 72 | 1:30 min | |
| 1 | 72 | 5 min |
| 1 | 8 | inf |
Results:
- Amplification worked very well, we had bright bands on the right height.
- Bands were cut out and DNA purified using QIAquick Gel Extration Kit.
17-08-2013
Amplification from FS_01 to FS_16; 4.2 kb
- Reaction
(3x50µl)
| what | µl |
|---|---|
| Template pSB4K5 | 1 |
| FS_01: (1/10) | 2.5 |
| FS_16: (1/10) | 2.5 |
| Phusion Master Mix | 25 |
| DMSO | 2.5 |
| dd H2O | 16.5 |
- Conditions
| Biorad MyCycler | ||
|---|---|---|
| Cycles-PCR | temperature [°C] | Time [s] |
| 1 | 98 | 5 |
| 12 | 98 | 1 |
| 62 | 5 | |
| 72 | 1:30 min | |
| 1 | 72 | 5 min |
| 1 | 8 | inf |
Results:
- Amplification worked very well, we had bright bands on the right height.
- Bands were cut out and DNA purified using QIAquick Gel Extration Kit.
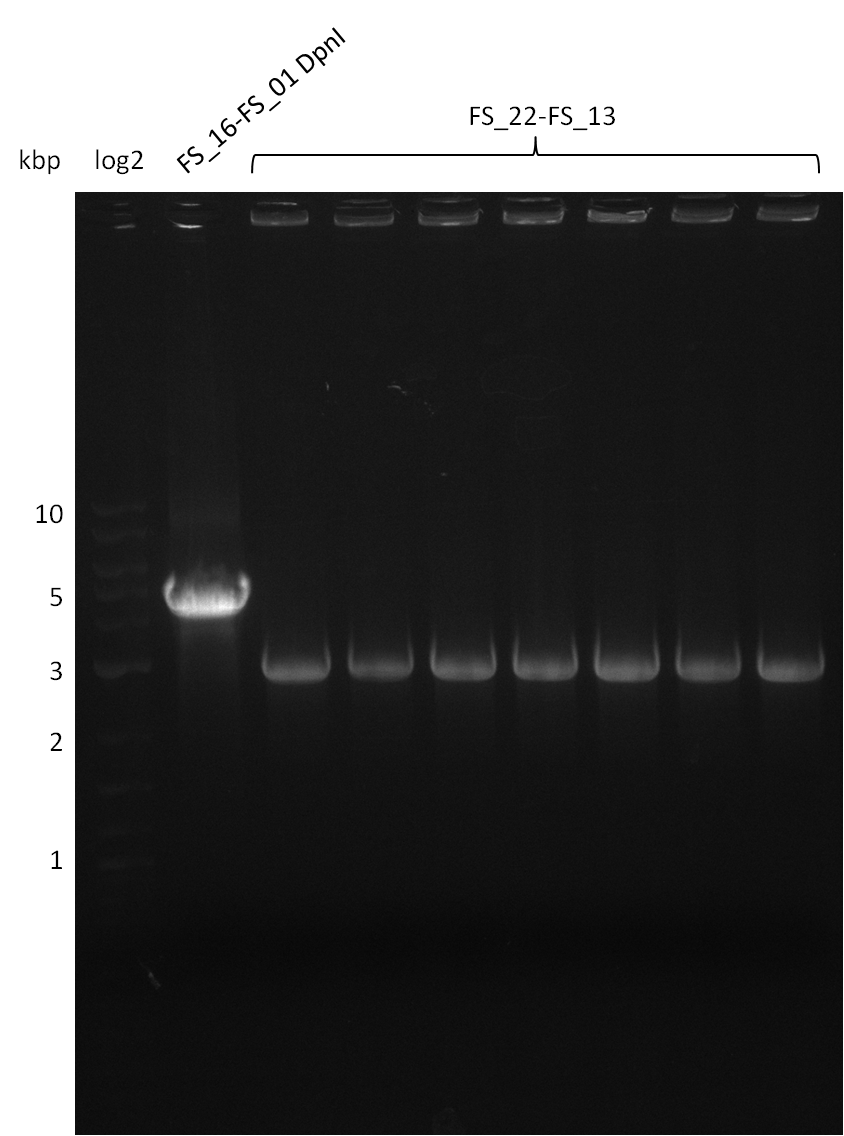
Restriction digest of pSB4K5 (FS_01 to FS_16; 4.2 kb; 17-08-2013) with DpnI
Incubation at 37°C for about 6 hours
| what | µl |
|---|---|
| FS_16 to FS_01 (17-08-2013) | 30 |
| DpnI | 2 |
| CutSmart Buffer | 4 |
| dd H2O | 4 |
Results:
- Bands were cut out and DNA purified using QIAquick Gel Extration Kit.
18-08-2013
Amplification from FS_01 to FS_16; 4.2 kb
- Reaction
(3x50µl)
| what | µl |
|---|---|
| Template pSB4K5 | 1 |
| FS_01: (1/10) | 2.5 |
| FS_16: (1/10) | 2.5 |
| Phusion Master Mix | 25 |
| DMSO | 2.5 |
| dd H2O | 16.5 |
- Conditions
| Biorad MyCycler* | ||
|---|---|---|
| Cycles-PCR | temperature [°C] | Time [s] |
| 1 | 98 | 5 |
| 12 | 98 | 1 |
| 62 | 5 | |
| 72 | 1:30 min | |
| 1 | 72 | 5 min |
| 1 | 8 | inf |
Restriction digest of pSB4K5 (FS_01 to FS_16; 4.2 kb; 18-08-2013) with DpnI
Incubation at 37°C for about 6 hours
| what | µl |
|---|---|
| FS_16 to FS_01 (18-08-2013) | 30 |
| DpnI | 2 |
| CutSmart Buffer | 4 |
| dd H2O | 4 |
Results:
- Digested fragment was run on a gel.
- Bands were cut out and DNA purified using QIAquick Gel Extration Kit.
 "
"