Team:Heidelberg/Templates/Indigoidine week11
From 2013.igem.org
(Created page with " ===Indigoidine production with pKH1 II (Konrad)=== ==== fragment amplification ==== ==> f1: bpsA (all) * 14.6 µl H2O * 25 µl Phusion Flash * 2x 0.5 µl Primer (NI01,NI06)...") |
|||
| Line 30: | Line 30: | ||
|} | |} | ||
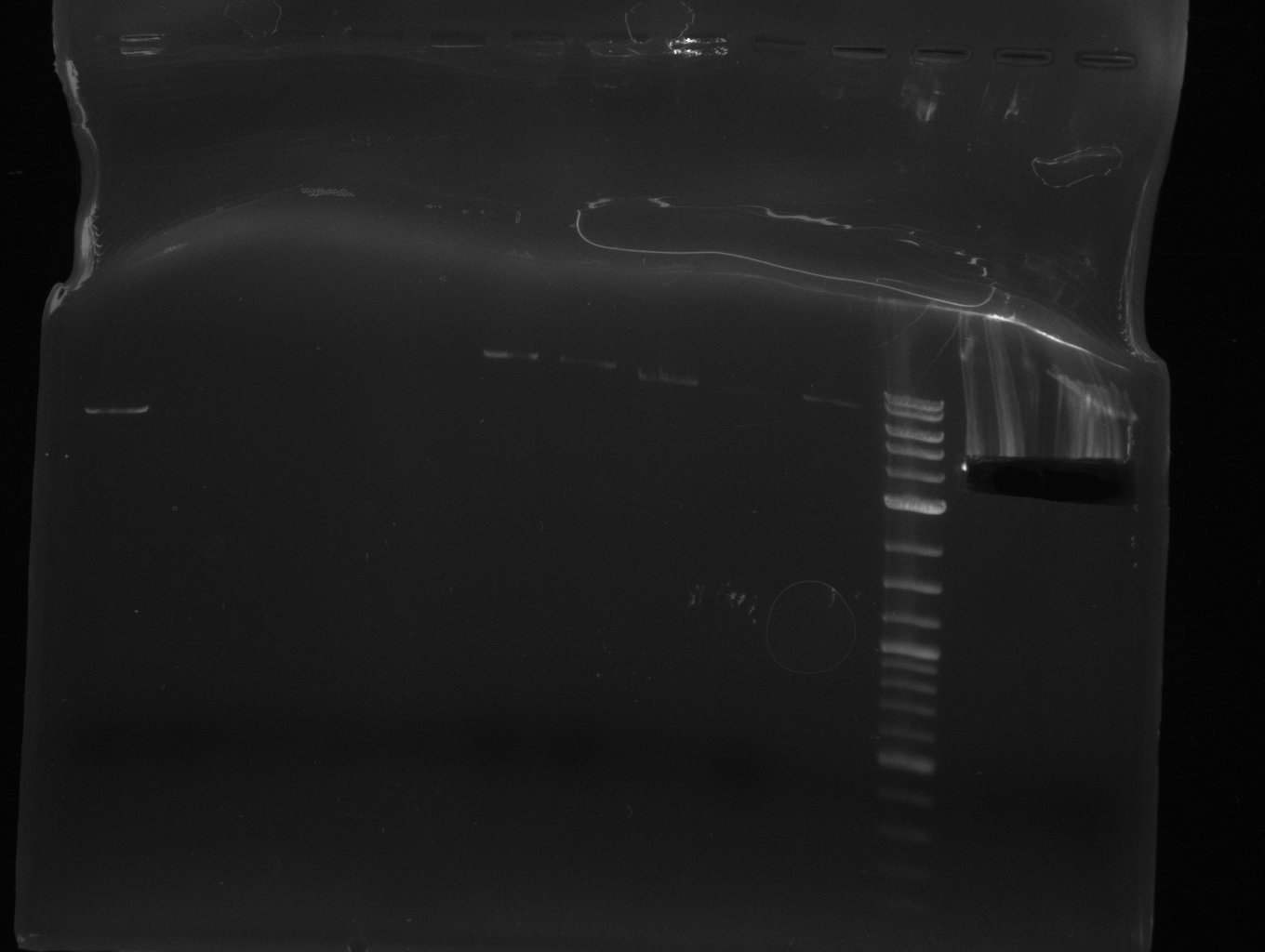
| - | [[File: | + | [[File:Heidelberg_2013-07-03 DelHf1a DelHf1b DelHf2 pSB6A1.jpg|400px|thumb|left|PCR for amplification of bpsA (all); lane 11: marker, lane 12 and 13: f1:BpsA (all), rest=see Del |
run at 135 V, 0.8 % gel (TAE); wanted amplicon size is: | run at 135 V, 0.8 % gel (TAE); wanted amplicon size is: | ||
bpsA: ~3.8 kbp]] | bpsA: ~3.8 kbp]] | ||
| - | [[File: | + | [[File:Heidelberg_20130703 BpsA cut.jpg|400px|thumb|right|Bands cut out of PCR (amplification of bpsA (all))]] |
<br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> | <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> | ||
| Line 65: | Line 65: | ||
|} | |} | ||
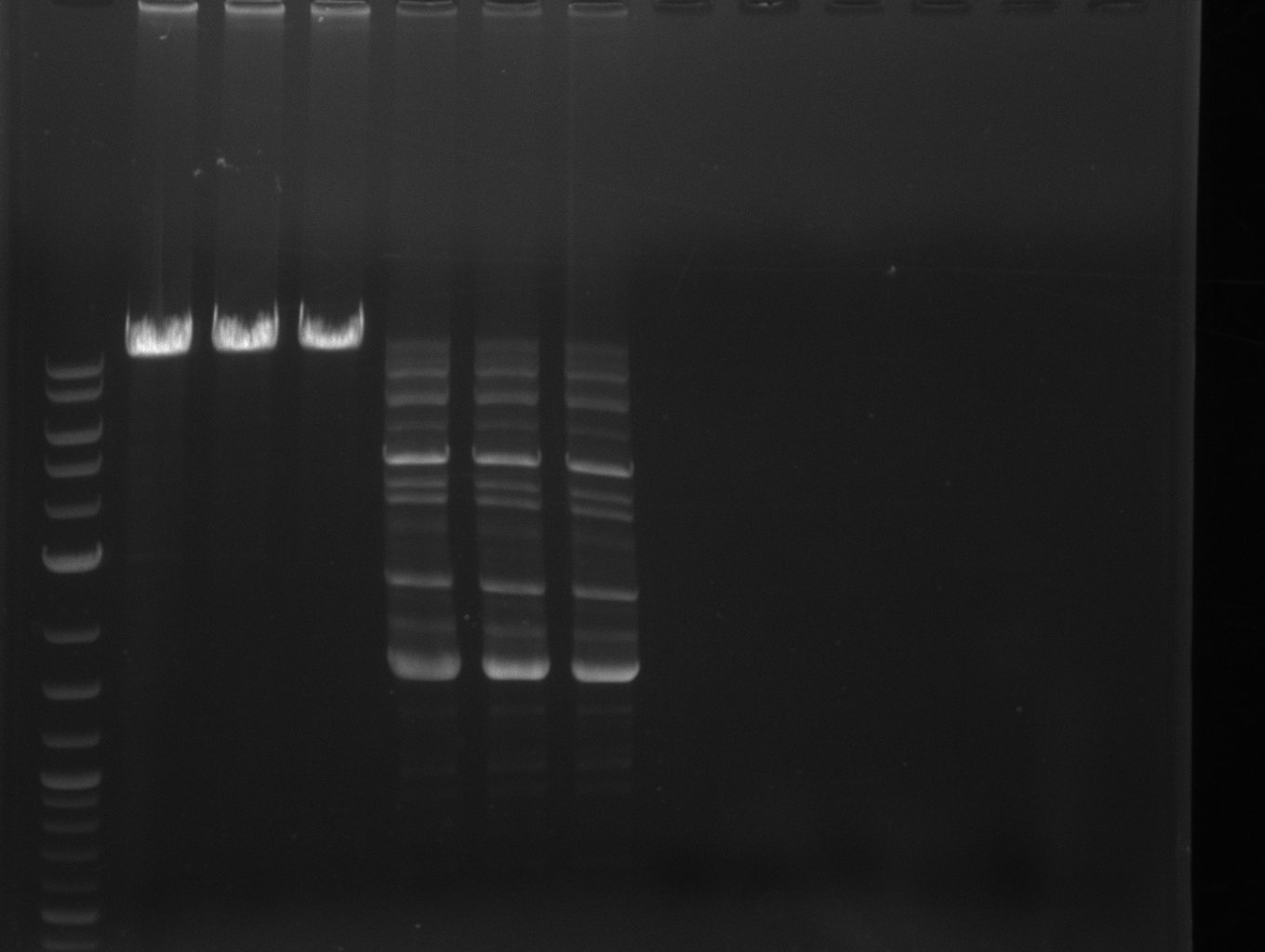
| - | [[File: | + | [[File:Heidelberg_20130703 fusion-svp+bpsA M DelH1a.jpg|400px|thumb|left|PCR for fusion of bpsA (all) and svp: lane 3 & 4, |
lane 6: marker, rest=see Del | lane 6: marker, rest=see Del | ||
run at 135 V, 0.8 % gel (TAE); wanted amplicon size is: | run at 135 V, 0.8 % gel (TAE); wanted amplicon size is: | ||
bpsA and svp: ~4.6 kbp]] | bpsA and svp: ~4.6 kbp]] | ||
| - | [[File: | + | [[File:Heidelberg_20130703 fusion-svp+bpsA(cutted) M DelH1a.jpg|400px|thumb|right|PCR for fusion of bpsA (all) and svp, |
cutted]] | cutted]] | ||
| Line 85: | Line 85: | ||
==> f7: pSB1C3 (linear.) with our Phusion MM and Phusion MM of SYNtheSYS (old, don't know if functional...) | ==> f7: pSB1C3 (linear.) with our Phusion MM and Phusion MM of SYNtheSYS (old, don't know if functional...) | ||
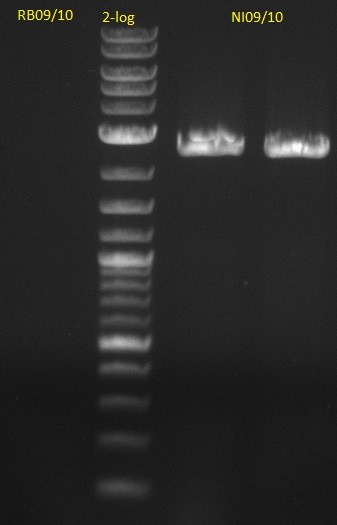
| - | [[File: | + | [[File:Heidelberg_20130704 M DelA-F DelF-G.jpg|400px|thumb|right|PCR for amplification of pSB1C3: lane 10 & 11: our fusion MM, |
lane 12 & 13: Synthesys fusion MM, lane 1: marker, rest=see Del Rest, | lane 12 & 13: Synthesys fusion MM, lane 1: marker, rest=see Del Rest, | ||
| Line 142: | Line 142: | ||
|} | |} | ||
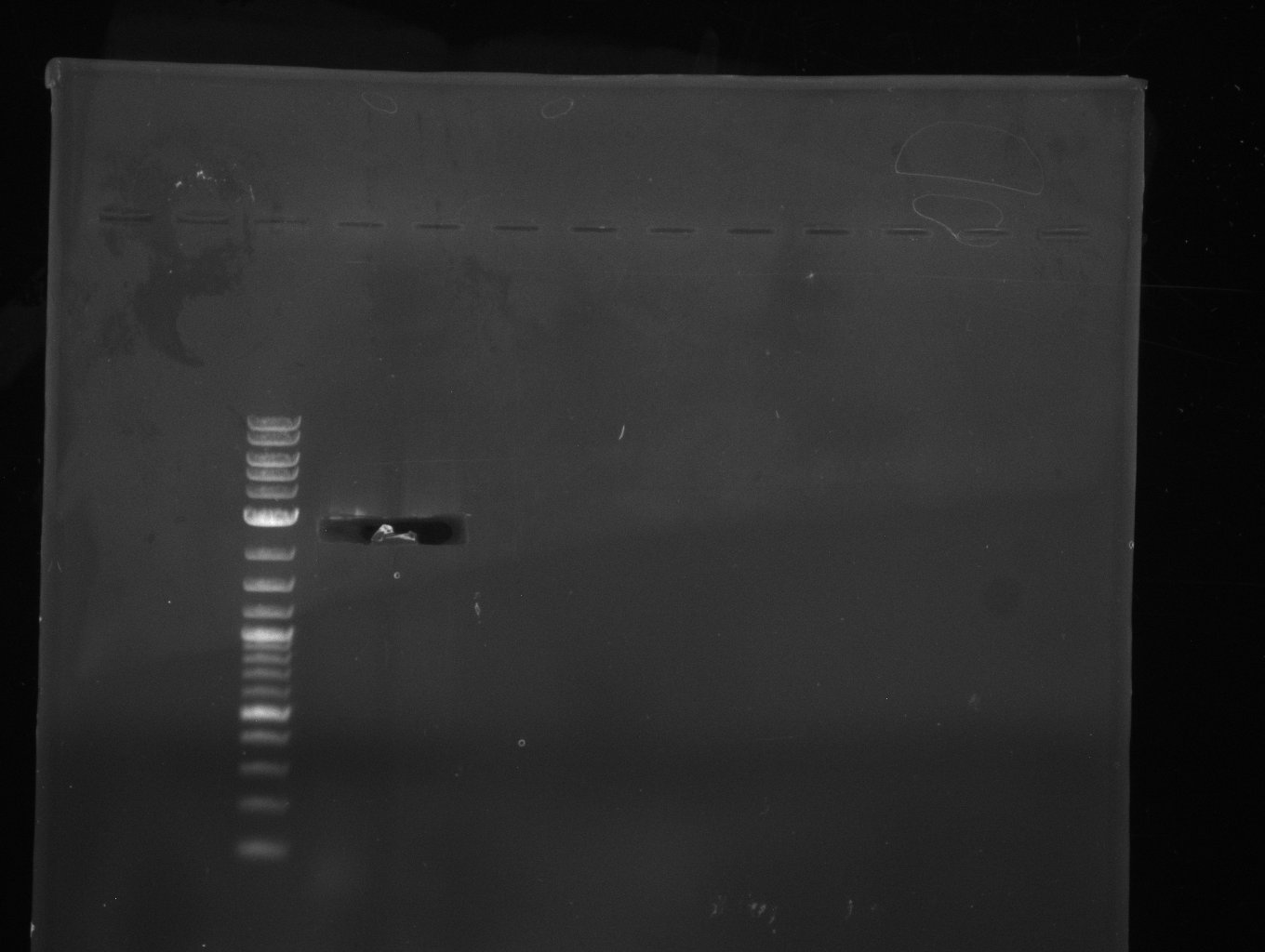
| - | [[File: | + | [[File:Heidelberg_20130704 pSB1C3.jpg|200px|thumb|left|PCR for amplification of pSB1C3: lane 3 & 4, lane 1=marker, rest=see |
Indigoidine Streptomyces, | Indigoidine Streptomyces, | ||
run at 135 V, 0.8 % gel (TAE); wanted amplicon size is: | run at 135 V, 0.8 % gel (TAE); wanted amplicon size is: | ||
bpsA: ~3.8 kbp]] | bpsA: ~3.8 kbp]] | ||
| - | [[File: | + | [[File:Heidelberg_20130704 pSB1C3 cut.jpg|400px|thumb|right|Bands cut out of PCR (amplification of pSB1C3)]] |
<br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> | <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> <br\> | ||
| Line 208: | Line 208: | ||
==> for amplification of fusion fusion product 03.07 (bpsA + svp) using Phusion Flash HF | ==> for amplification of fusion fusion product 03.07 (bpsA + svp) using Phusion Flash HF | ||
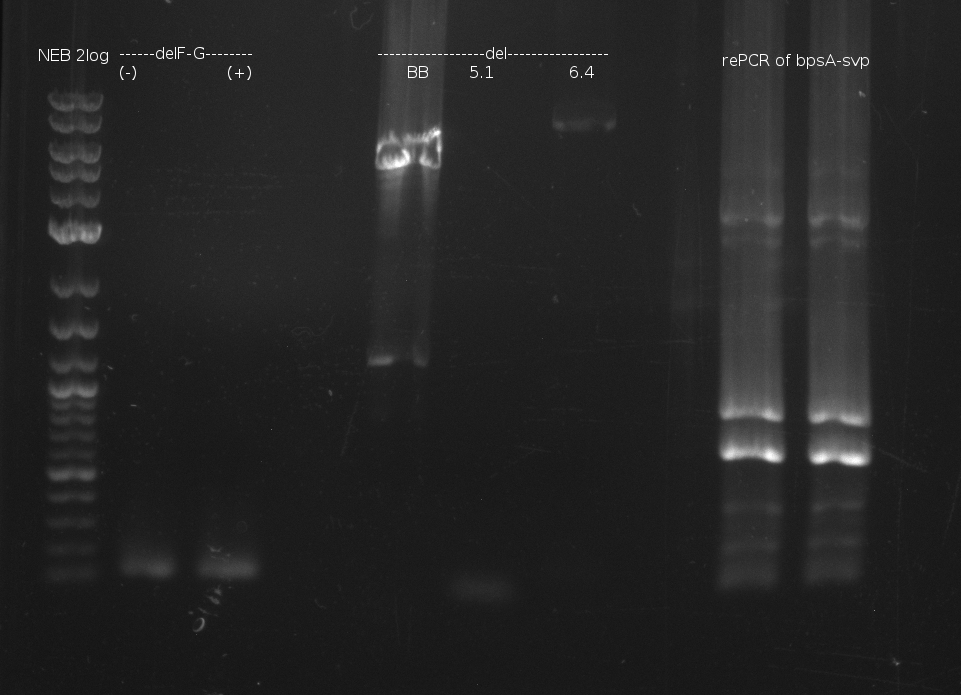
| - | [[File: | + | [[File:Heidelberg_060713_delRest_rePCR.png|500px|thumb|Gel electrophoresis of DelRest relevant fragment, and of rePCR of |
bpsA-svp of 03.07. fusion PCR: shows two bands at too small fragment size and additionaly unspecif bands]] | bpsA-svp of 03.07. fusion PCR: shows two bands at too small fragment size and additionaly unspecif bands]] | ||
| Line 304: | Line 304: | ||
|} | |} | ||
Gel | Gel | ||
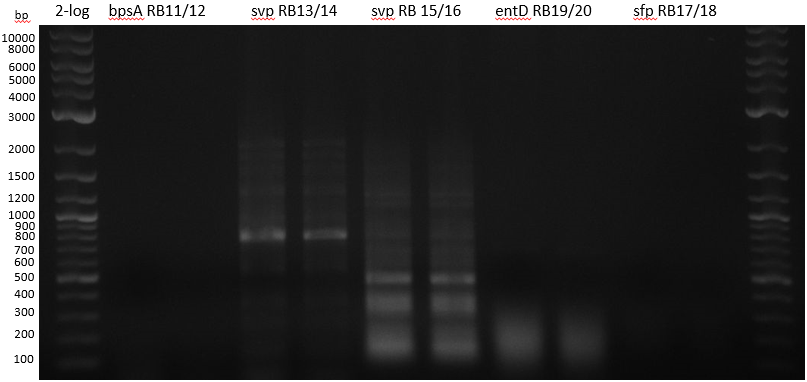
| - | :[[ | + | :[[File:Heidelberg_20130712_PPTases.PNG|400px|2-log; bpsA; svp 13/14; svp 15/16; entD; sfp]] |
Gradient PCR with both Streptomyces under same conditions to validate PCR parameters. | Gradient PCR with both Streptomyces under same conditions to validate PCR parameters. | ||
Phusion Flash HF 20 ul (10 ul MM; 2 ul Primer 10 mM; 2 uL template (cell pellet from GYM 48 h liquid culture; 6 ul | Phusion Flash HF 20 ul (10 ul MM; 2 ul Primer 10 mM; 2 uL template (cell pellet from GYM 48 h liquid culture; 6 ul | ||
| Line 323: | Line 323: | ||
|} | |} | ||
Gel | Gel | ||
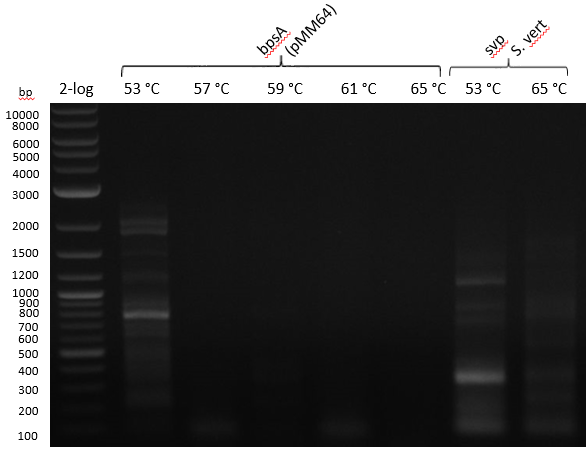
| - | :[[ | + | :[[File:Heidelberg_20130714_strepto.PNG|400px|bpsA 53-57-59-61-65; svp 53-65]] |
===Results and Discussion=== | ===Results and Discussion=== | ||
Revision as of 00:45, 5 October 2013
Contents |
Indigoidine production with pKH1 II (Konrad)
fragment amplification
==> f1: bpsA (all)
- 14.6 µl H2O
- 25 µl Phusion Flash
- 2x 0.5 µl Primer (NI01,NI06)
- 0.4 µl template pMM64
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 66 | 5 | |
| 72 | 120 | |
| 1 | 72 | 420 |
| 1 | 4 | inf |
- Gel purification (50µl): by accident did not take 3 but 1 Volume buffer QG
- Labelling fragements: 1 I and 1 II (from different lanes)
fusion PCR
==> for fusion of both fragments bpsA and svp using Phusion Flash HF
- 19.8 µl H2O
- 25 µl Phusion Flash MM
- 2x 1.0 µl Primer (NI01,NI08)
- 2.6 µl template (f1:bpsA)
- 0.6 µl template as 1:10 dilution (f6:svp)
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 72 | 70 | |
| 1 | 72 | 180 |
| 1 | 4 | inf |
- Gel purification (50µl): by accident did not take 3 but 1 Volume buffer QG
- Labelling fragments: 1 I and 1 II (from different lanes)
Amplification pSB1C3 (f7)
==> f7: pSB1C3 (linear.) with our Phusion MM and Phusion MM of SYNtheSYS (old, don't know if functional...)
(add items in this order)
- 14.6 µl H2O
- 25 µl Phusion MM
- 2x 0.5 µl Primer (NI09,NI10)
- 0.4 µl template (pSB1C3 with J04450 (pJM03))
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 30 |
| 30 | 98 | 10 |
| 72 | 90 | |
| 1 | 72 | 420 |
| 1 | 4 | inf |
-->Hot start
RESULT: no product neither with Synthesys fusion nor our fusion
==> f7: pSB1C3 (linear.) with Fusion Flash
(add items in this order)
- 14.6 µl H2O
- 25 µl Phusion Flash
- 2x 0.5 µl Primer (NI09,NI10)
- 0.4 µl template (pSB1C3 with J04450 (pJM03))
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 72 | 40 | |
| 1 | 72 | 420 |
| 1 | 4 | inf |
fusion PCR
==> for fusion of both fragments bpsA and svp using Phusion Flash HF
- 19.8 µl H2O
- 25 µl Phusion Flash MM
- 2x 1.0 µl Primer (NI09,NI10)
- 5 µl template (f1:bpsA I,03.07.13)
- 0.6 µl template as 1:10 dilution (f6:svp)
--> wrong primers, should have taken NI01 and NI08
--> more than 50µl as not enough bpsA template was left
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 72 | 70 | |
| 1 | 72 | 180 |
| 1 | 4 | inf |
Re-PCR of fusion (bpsA+svp)
==> for amplification of fusion fusion product 03.07 (bpsA + svp) using Phusion Flash HF
- 18 µl H2O
- 25 µl Phusion Flash MM
- 2x 1.0 µl Primer (NI09,NI10)
- 5 µl template (fusion bpsA+svp,03.07.13)
--> wrong primers, should have taken NI01 and NI08
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 72 | 70 | |
| 1 | 72 | 180 |
| 1 | 4 | inf |
Re-PCR of fusion (bpsA+svp)
==> for amplification of fusion fusion product 03.07 (bpsA + svp) using Phusion Flash HF
- 19 µl H2O
- 25 µl Phusion Flash MM
- 2x 0.5 µl Primer (NI01,NI08)
- 5 µl template (fusion bpsA+svp,03.07.13)
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 72 | 70 | |
| 1 | 72 | 180 |
| 1 | 4 | inf |
Preparations
- prepare liquid culture of Rosetta + pMM64 + pMM65 in 2 ml LB with Amp + Kan for plate oculation
- prepare liquid cultures of TOP10 + pMM64/65 for minipreps: 3 ml TB + Amp/Kan
- prepare liquid cultures for induction test: 3 ml LB + appropiate AB
- BAP1
- BAP1 + pMM64 (Amp)
- BAP1 + pMM64 + pMM65 (Amp + Kan)
- TOP10 + pJH1 (Kan)
Re-PCR of fusion (bpsA+svp)
Ralf
PCR from glycerol stock S. lavendulae with Fermentas; Thermoscientific and NEB Taq 50 ul
- MM 25 ul, 7,5 ul primer RB7/8 100 mM, 5 ul water, 5 ul culture
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 95 | 300 |
| 30 | 95 | 30 |
| 50 | 40 | |
| 70 | 300 | |
| 1 | 70 | 300 |
| 1 | 4 | inf |
- S. verticillus and S lavendulae in 50 ml YEME medium for 46 h at 28 °C.
- filamentous growth and spherical colonies in S. lavendulae flask after 1 day -> contamination?!
- no growth in S. verticillus flask after 18 h
- prepare plates
colony PCRs
| 1 bpsA S. lav | 2 svp S. vert | 3 svp S. vert | 4 entD MG1655 | 5 sfp BAP1 | |
|---|---|---|---|---|---|
| water | 10 | 10 | 10 | 10 | 10 |
| Phusion Flash MM | 25 | 25 | 25 | 25 | 25 |
| Primer | à 5 ul RB11/12 | à 5 ul RB13/14 | à 5 ul RB15/16 | à 5 ul RB19/20 | à 5 ul RB17/18 |
| template | colony pick from plate | cell pellet liquid culture | cell pellet liquid culture | cell pellet glycerol
stock||colony from plate | |
| biometra | BioRAD test machine | ||||
colony PCRs 50 ul Phusion flash (25 ul + 5 ul Primer 10 mM + 10 ul water
- bpsA - S. lav (Takahashi) RB11/12
- svp - S. vert (Taka Primer) RB13/14
- svp - S. vert (Sanchez) RB15/16
- entD - MG1655 (Lambalot) RB19/20
- sfp - BAP1 RB17/18
biometra
| 1 | 98 | 120 |
| 98 | 1 | |
| 65 | 5 | |
| 72 | 20/60 | |
| 12 | - |
Gel
Gradient PCR with both Streptomyces under same conditions to validate PCR parameters. Phusion Flash HF 20 ul (10 ul MM; 2 ul Primer 10 mM; 2 uL template (cell pellet from GYM 48 h liquid culture; 6 ul
H2O)
| 1 | 98 | 120 |
| 30 | 98 | 1 |
| 53-57-59-61-65 | 5 | |
| 72 | 60 | |
| 12 | - |
Gel
 "
"