Team:NCTU Formosa/project
From 2013.igem.org
Contents |
Future Work
The multiple regulated-system we have created in this project consists of two different parts: the light induced part and the dark induced part. Each part regulates two different genes, resulting in a total of four genes that can be regulated. We tend to regulate more genes by employing more light sensing promoters. By adding each light sensor, we would be able to regulate two more different genes by employing regulation mechanism of 37°C RBS and sRNA.
Application
Taiwan is renowned for Phalaenopsis, producing more than 35 million Phalaenopsis each year. There is no doubt that Taiwan has become "The Kingdom of Phalaenopsis". The technique we usually use to grow Phalaenopsis is plant tissue culture, thus we intend to apply it to our system.
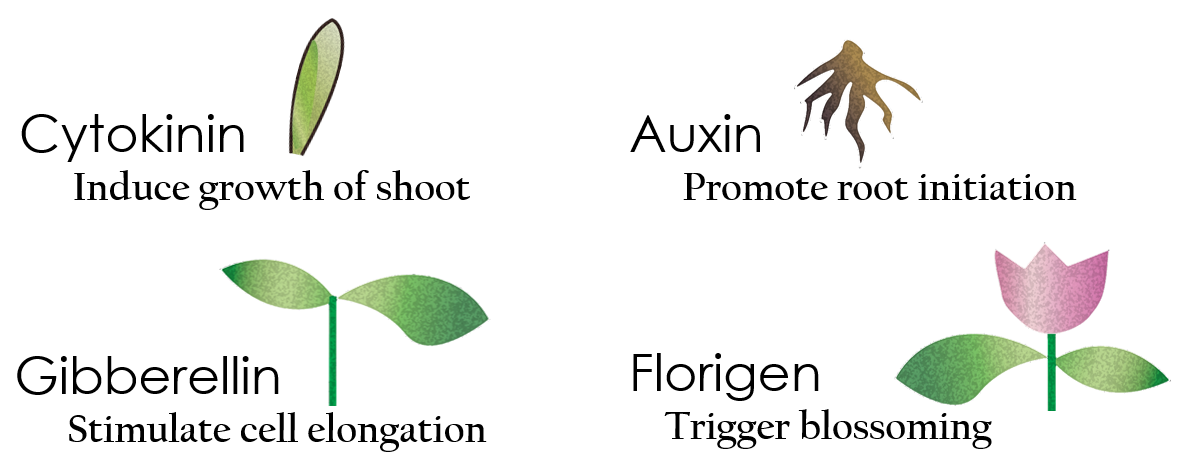
Tissue culture is the growth of tissues or cells separated from the organism. This is typically facilitated via use of a liquid, semi-solid or solid growth medium, such as broth or agar. Moreover, hormones are added to the growth medium purposed to what plants need during growth period. However, the hormones needed during each growing state are not the same. So, we need to figure out a way to generate multiple hormones. By adding our engineered E.coli to the growth medium, the plants can gain multiple hormones secreted by E. coli under different circumstances. We chose four common hormones as our genes, Auxin, Cytokinin, Gibberellin, and Florigen.
Since Florigen(known as theFT protein) is the protein which isn't produced by E.coli naturally, there isn't any secretion system in wild type E. coli to transport Florigen outside of the cells. Though secretion by cell lysis is a possible method to solve this problem, we still seek for other ways in order not to let our E. coli die during the secretion process. Thus, we ought to create a secretion system which can help Florigen pass through the inner and outer membrane of E. coli. We have found a system from the 2012 Kyoto iGEM team called Twin Arginate Translocation (Tat) pathway, which can help send Florigen through the inner membrane to periplasm. In Tat pathway, a Tat transporter recognizes TorA signal. Proteins possessing a TorA signal at their N terminals can permeate into the periplasm. Moreover, the 2012 Kyoto iGEM team also found out that kil protein from λ phage can make holes on the outer membrane, which then transports Florigen outside cells. In other words, with the kil protein gene fused with the torA and FT protein gene in our biobrick, we should be able to successfully transport Florigen out of the E. coli.
The use of Auxin is to induce the growth of shoot, but an excess amount of Auxin would kill the plant. We want to reach the maximum amount that will promote the plant’s growth but without killing the plant. After researching, the Imperial College London 2011 iGEM team also has the same problem. They chose the native Auxin indole-3-acetic acid(IAA) as their solution. Compared to the former, the synthetic Auxin is more stable that it can preserve for weeks. However, an excess amount of IAA could be toxic. To ensure the efficiency but at the same time not excessive, they built a model to reach the maximum amount.
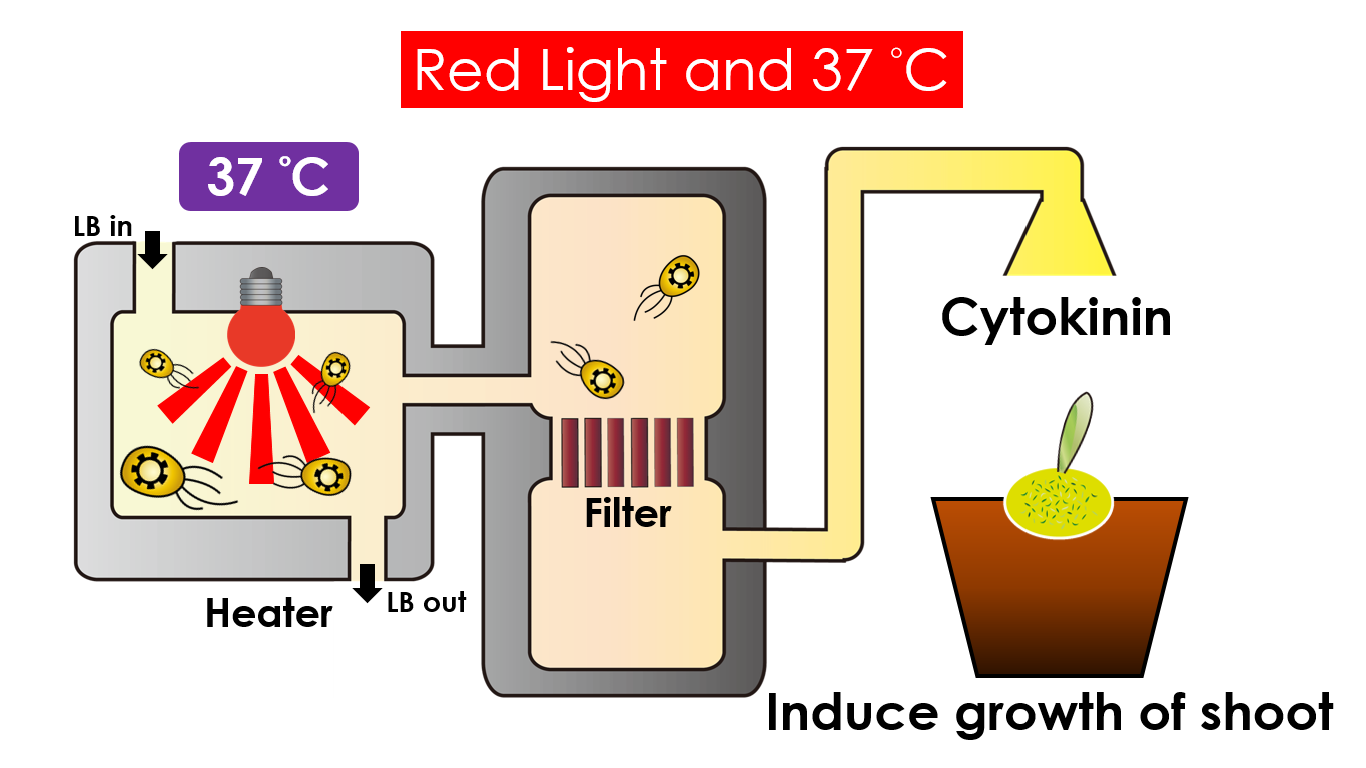
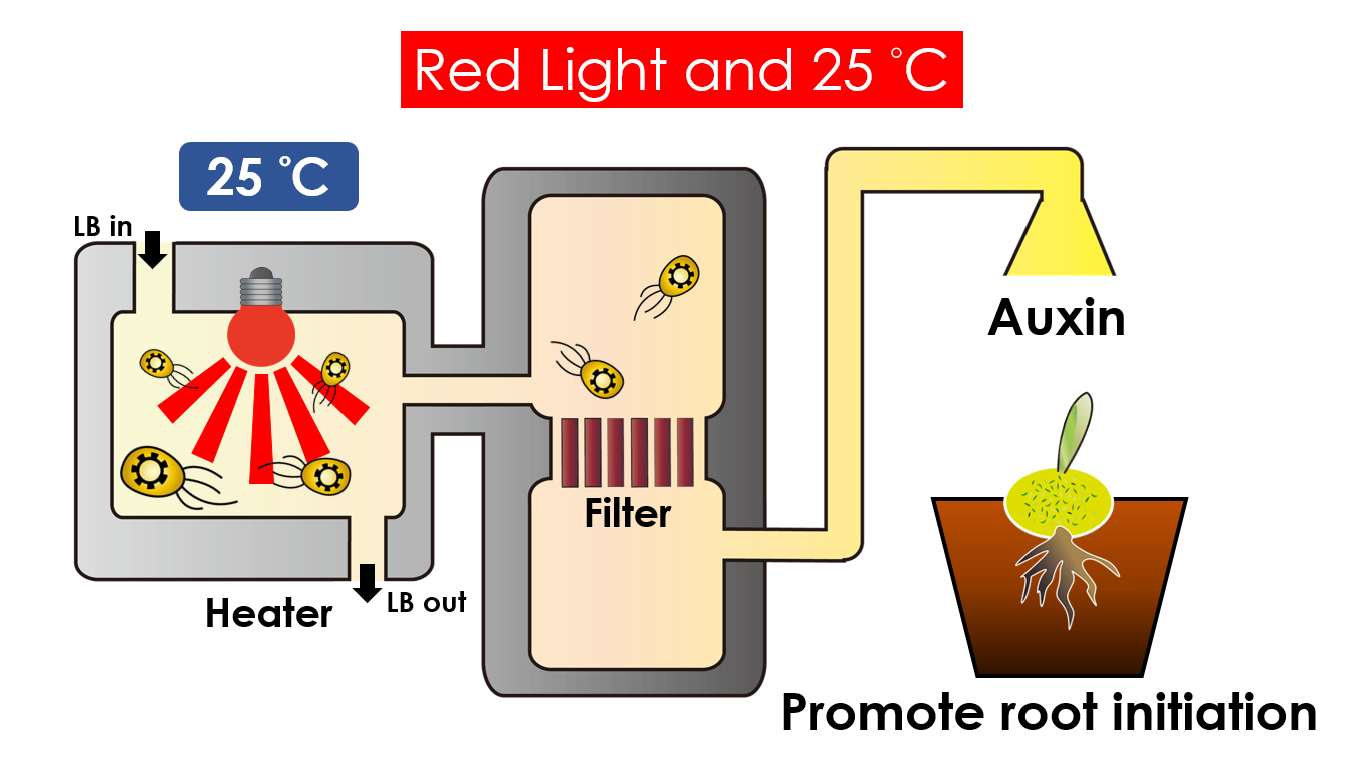
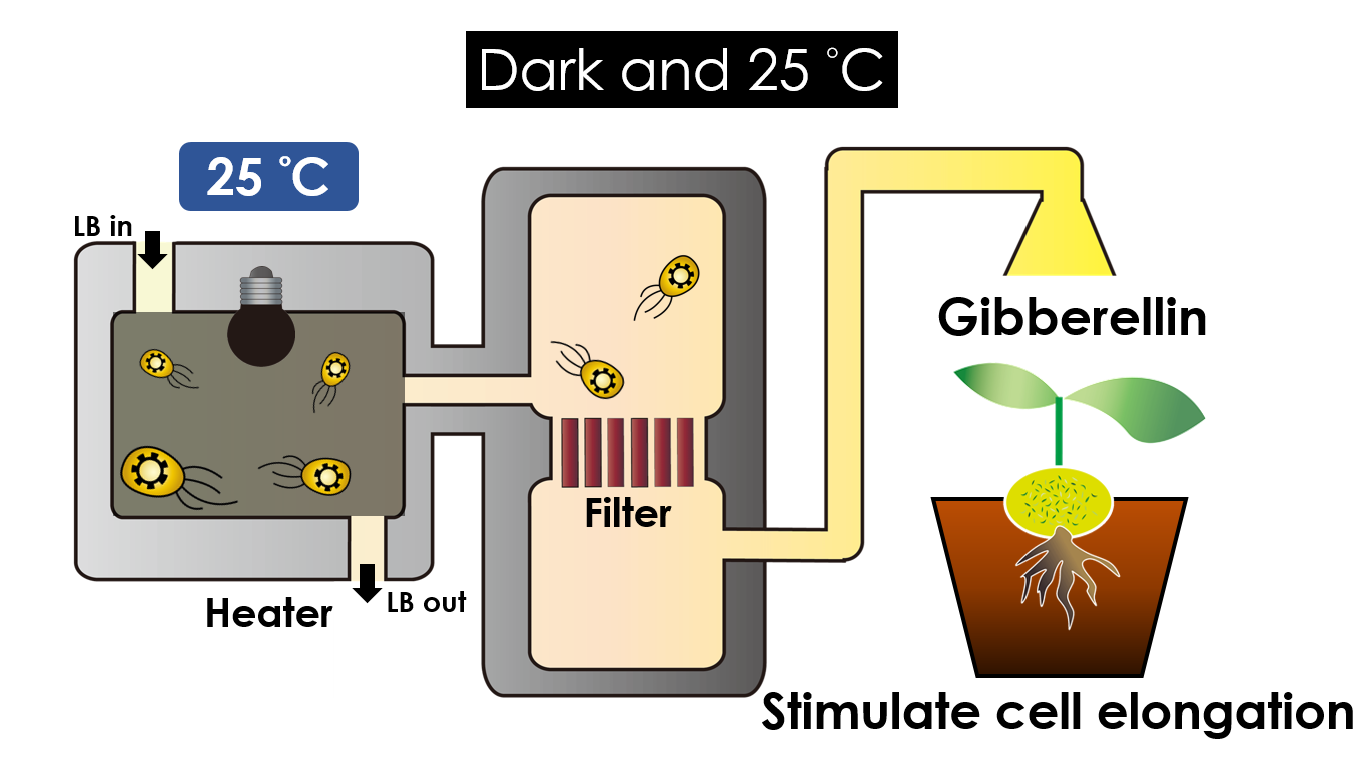
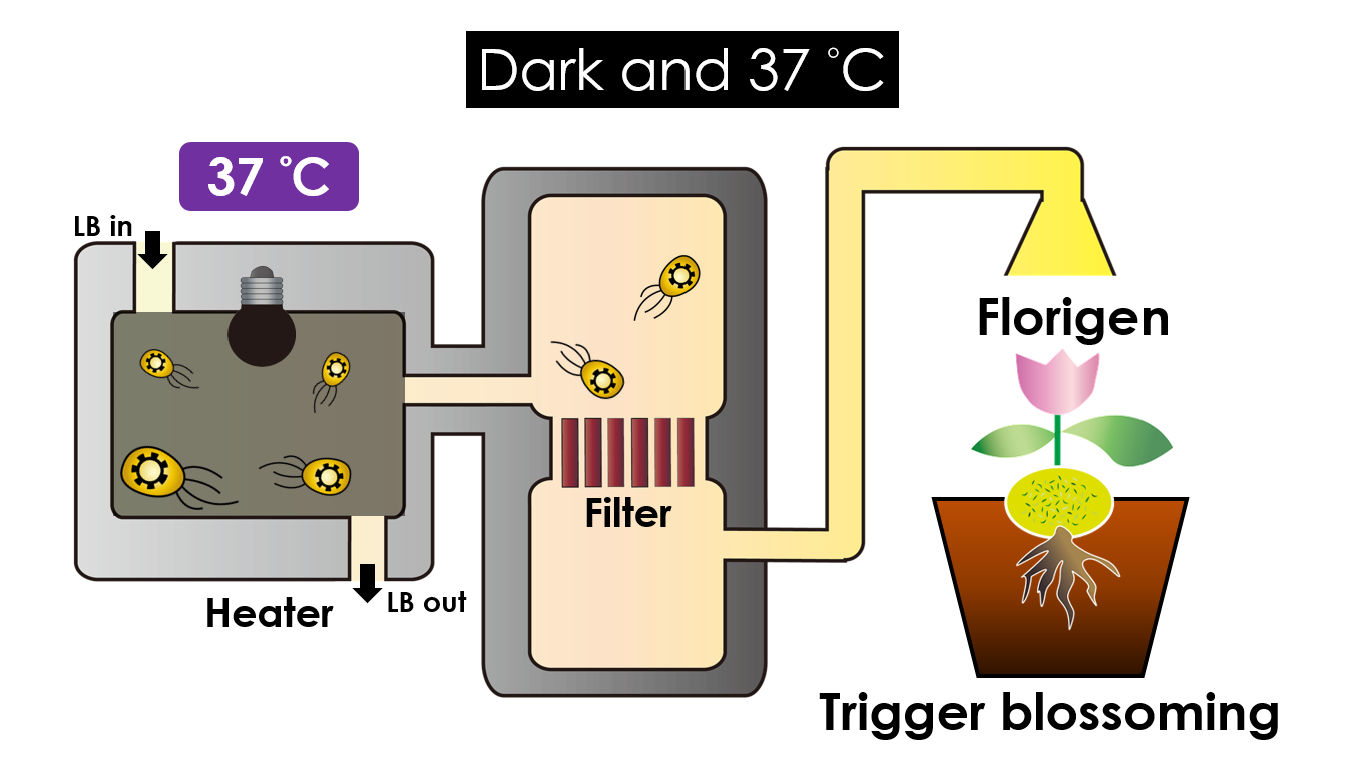
We intend to carry out our application with the device beneath. Cultured in the growth medium circulation, our engineered E. coli can absorb enough nutrition to express the desired genes properly. We also use a semi-permeable membrane, which allows hormones but not bacteria themselves to pass through, separating the bacteria from plant tissues. Thus, we keep the tissue culture medium separately.
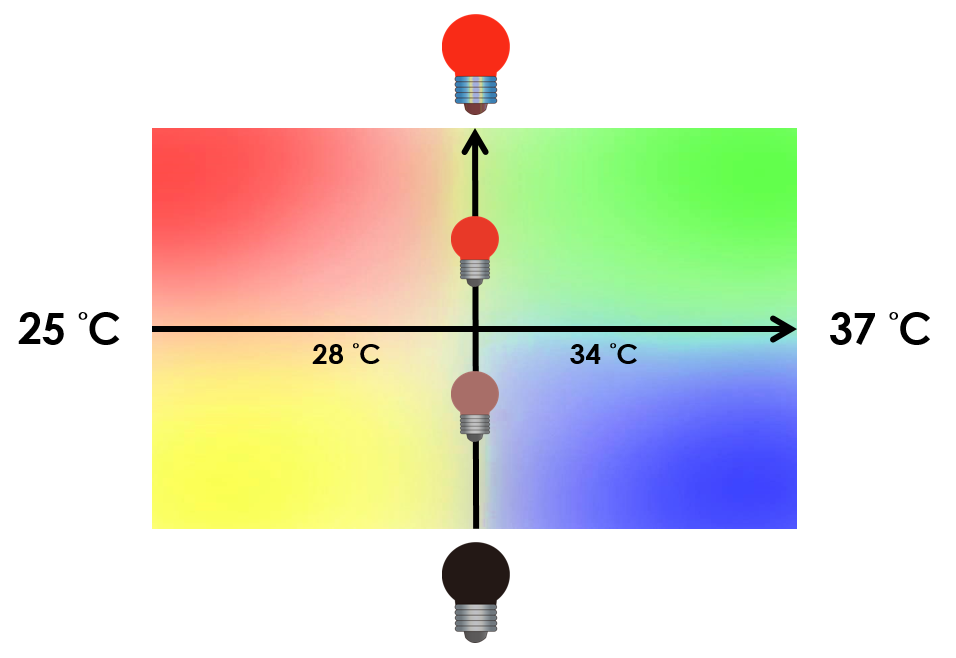
Under red light and 37°C, Cytokinin will be produced. Cytokinin is a plant growth substance which can primarily induce cell growth and differentiation in plant roots and shoots. It can decrease apical dominance which will make the main stem grow better compared to other stems. In this way, all the stems are able to become stronger. Cytokinin can also signal lateral bud growth, which will make the plant bushier.
Under red light and 30°C, Auxin will be produced. Auxin can coordinate development from cellular, organs to the whole plant. Auxin molecules in cells may cause direct responses by stimulation and inhibitions of certain gene expressions. On the cellular level, Auxin is important for cell growth. It can induce axial elongation in roots and lateral expansion which can make the roots swell. For the whole plant level, it affects the shape of the plants. Because of the different functions, each organ has uneven Auxin concentration and causes specific shapes.
Under dark and 30°C, Gibberellin will be produced. Gibberellin is important in the elongation of cells. It causes the extension of stems by inducing cell divisions and elongations. A special characteristic of it is that it can produce more mass when the plant is exposed to cold temperature.
Under dark and 37°C, Florigen will be produced. Florigen is the key hormone to trigger plant blossoming. We use a promoter that can promote transcription of the FT gene, which then translate into FT proteins. FT proteins will then be transported via the phloem to the shoot apical meristem, where it will interact with the FD protein(transcription factor) to activate floral identity, thus inducing flowering.
The next big thing
On the other hand, we tend to optimize our system to achieve subtle control of the four gene expressions. We might be able to do this by taking the values between the limits we have set. Instead of 30°C and 37°C , we tend to take the values between, so we can create more conditions under which different level of expressions can be achieved. We can also vary the intensity of red light to control the level of expression. For instance, we can get different pigment outputs by tuning the temperature and varying the intensity of red light. The ultimate goal is to precisely control the level of expression of each gene.
因為Pred的直接性,在未來可以拿來測試被紅光、藍光的影響度?
Reference
- Team : Imperial_College_London -2011.igem
- Team : Kyoto -2012.igem
</div></div>
 "
"