Team:SydneyUni Australia/Project/Design
From 2013.igem.org

Gibson
Gibson Assembly *should* make for a much easier, simpler, rapid assembly of different genes than conventional PCR and cloning. There is also more flexibility for optimisation through gene synthesis.
- The assembly works on fragments of DNA with ~30bp of overlapping sequence, which is exposed as 5’ single-stranded overhangs by an exonuclease. A ligase joins the overlapping regions and a polymerase fills in any gaps left by the exonuclease. These enzymes can all work together in a single reaction tube with many different overlapping fragments, making the assembly a very, very simple activity. Gibson Assembly is based on the older technique of PCR Assembly, with the similar reliance on the initial construction of 200+bp fragments from smaller oligos, but with a greater degree of sequence fidelity due to less polymerase activity.
- Here’s a great introduction from IDT’s magazine, DECODED, and a more in-depth webinar.
- If you’re historically-minded or want more detail, try the original paper in which Gibson Assembly was described - or one of the coolest and most famous applications of Gibson, building a synthetic genome.
Design
Choice of genes
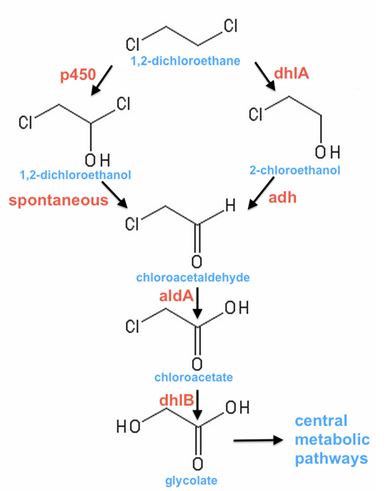
Mox, chloroethanol dehydrogenase
- In sketches of our project (February-March), we planned to use Mox as the alcohol dehydrogenase converting chloroethanol to chloroacetaldehyde. After a little more research we discovered that this enzyme requires the co-factor PQQ, and unfortunately for us, this co-factor requires many genes for its synthesis, which would have made our constructs too complex (Khairnar et al., 2003).
- Mox had seemed like an obvious choice because it would be sourced from Xanthobacter autotrophicus GJ10, the most well-documented DCA-degrader (Janssen et al., 1987)
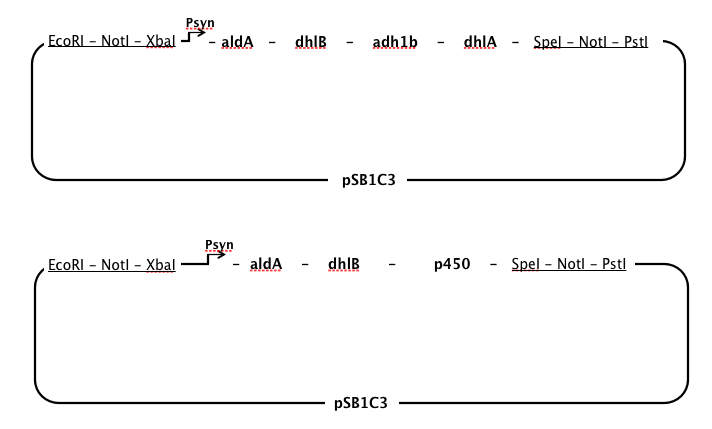
aldA, chloroacetaldehyde dehydrogenase, dhlB, haloacid dehalogenase, and dhlA, haloalkane dehalogenase
- We used a sequence for aldA from Xanthobacter autotrophicus GJ10, but used sequences for dhlB and dhlA from a different strain (Xanthobacter autrophicus) EL4, which was isolated and characterised in the Coleman lab. These two genes had been identified and cloned into pUC19 by Jake Munro, a research assistant in the Coleman lab. When our Gibson Assembly ran into problems, we continued to work with dhlB and dhlA from EL4 in our lab and submitted these two genes as parts.
- These genes are shared by a few different species of bacteria that degrade DCA (Janssen et al., 1994), and most have been conventionally characterised by extraction and heterologous expression of a single gene at a time.
ToMO, toluene-o-xylene monooxygenase
- Early in our project we showed that ToMO can degrade DCA. This would not only eliminate the need for something like PQQ-synthase, but also make for a shorter, less complex pathway involving just 3 enzymes (a monooxygenase, dehydrogenase and dehalogenase).
- We initially planned to synthesise ToMO, allowing us to remove forbidden restriction sites in the process (EcoRI and PstI). However, the sheer size of the monooxygenase cluster (~5kb) made it prohibitively expensive.
- The gene we worked with initially has been extensively used for protein engineering by Varder & Wood (2005).
p450, cytochrome p450 monooxygenase
- Early in 2013 it was discovered that a monooxygenase from a Polaromonas sp. (JS666) was responsible for the initial steps of DCE and DCA degradation by heterologous expression in E. coli (Nishino et al., 2013). Evidence suggests that this enzyme effectively substitutes for ToMO in our pathway. Due to the shorter length of the three-gene p450 complex (~3kb) we decided to synthesise this enzyme instead of ToMO.
- The strain that carries this enzyme was first isolated from chloroethene contaminated sites by our primary supervisor, Nick Coleman, in the Stone Age (Coleman et al., 2002).
adh1b2, human liver alcohol dehydrogenase
- Human liver alcohol dehydrogenases have been shown to be active on a broad range of substrates, including haloalcohols and haloaldehydes (Blair & Vallee, 1966). People have expressed these enzymes in E. coli before (eg, Zheng et al., 1993) using cDNA, but we were able to borrow a modified mRNA sequence from GenBank (BC033009.2). Since we were also able to choose between many types and classes of human liver enzyme, we picked the one with the greatest turnover on ethanol as substrate (Edenberg, 2007).
Order of gene expression
- We chose to have a single, strong constitutive promoter in our pathway, so it made sense to place our genes in order from last to first in order that at every step in the pathway there would be an excess of the enzyme required for the substrate, thus minimising the build-up of toxic intermediates.
- However, chloroacetaldehyde appears to be the most toxic of the intermediates produced while DCA is degraded (Janssen et al., 1994). It seemed necessary to position the enzyme specific to aldA immediately after the promoter to ensure that chloroacetaldehyde was rapidly removed from the cell.
Optimising a promoter
- We used a single synthetic promoter region based on Plac, but including changes that other people have shown increase RNA polymerase binding. All the changes were made to maximise RNA polymerase recognition.
Ribosome binding sites
We added or modfied the same RBS (AAGGAGG) before all our genes, for a mix of these reasons:
- The natural sequences we borrowed were from diverse hosts and didn’t contain RBS optimal for E. coli.
- Sequences were found as annotated coding regions without an RBS.
- Sequences had apparently sub-optimal RBSs too close or distant to the start codon.
Restriction sites
We removed all restriction sites in our sequences that are forbidden within Registry parts (EcoRI, NotI, XbaI, SpeI, PstI). This had to be done while ensuring not to change codon usage, or change to an rare codon. We used this codon table.
Codon usage
An online application was used to identify codons rarely used by E. coli . Again, changes had to be made while ensuring not to change codon usage. All stop codons were converted to the more common variant and included as double-stops (TAATAA).
GC content and hairpins
- The template we used for Gibson Assembly was purchased as gBlocks from IDT with their iGEM discount.
- Some regions need to be avoided in the assembly of larger fragments (~500bp) from smaller oligos (60-120bp) during synthesis. These are regions contain extremes of GC content and regions that form small, six-base hairpins. We tried to avoid problems by running all our sequences through the gBlocks wizard before ordering and addressing any problems. However, the application failed to find a few problematic regions and these caused delay before the arrival of our gBlocks.
References
- Blair, A. H., & Vallee, B. L. (1966). Some Catalytic Properties of Human Liver Alcohol Dehydrogenase*. Biochemistry, 5(6), 2026-2034.
- Coleman, N. V., Mattes, T. E., Gossett, J. M., Spain, J. C. (2002) Biodegradation of cis-Dichloroethene as the Sole Carbon Source by a β-ProteobacteriumApplied and Environmental Microbiology, 68(6), 2726-2730.
- Edenberg, H. J. (2007). The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Research & Health, 30 (1), 5-13.
- Giacomini, A., Ollero, F. J., Squartini, A., & Nuti, M. P. (1994) Construction of multipurpose gene cartridges based on a novel synthetic promoter for high-level gene expression in Gram-negative bacteria. Gene, 144(1), 17-24.
- Janssen, D. B., Keuning, S., & Witholt, B. (1987). Involvement of a quinoprotein alcohol dehydrogenase and an NAD-dependent aldehyde dehydrogenase in 2-chloroethanol metabolism in Xanthobacter autotrophicus GJ10. Journal of General Microbiology, 133(1), 85-92.
- Janssen, D. B., Pries, F., & Van der Ploeg, J. R. (1994). Genetics and biochemistry of dehalogenating enzymes. Annual Reviews in Microbiology, 48(1), 163-191.
- Khairnar, N. P., Misra, H. S., & Apte, S. K. (2003). Pyrroloquinoline–quinone synthesized in Escherichia coli by pyrroloquinoline–quinone synthase of Deinococcus radiodurans plays a role beyond mineral phosphate solubilization. Biochemical and Biophysical Research communications, 312(2), 303-308.
- Liu, M., Tolstorukov, M., Zhurkin, V., Garges, S., Adhya, S. (2004) A mutant spacer sequence between -35 and-10 elements makes the Plac promoter hyperactive and cAMP receptor protein-independent. Proceedings of the National Academy of Sciences of the United States of America, 101(18), 6911-6916.
- Nishino, S. F., Shin, K. A., Gossett, J. M., Spain, J. C. (2013). Cytochrome P450 Initiates Degradation of cis-Dichloroethene by Polaromonas sp. Strain JS666 Applied and Environmental Microbiology, 79(7), 2263-2272.
- Ross, W., Aiyar, S. E., Salomon, J., Gourse, R.L. (1998) Escherichia coli promoters with UP elements of different strengths: modular structure of bacterial promoters. Journal of Bacteriology, 180(20), 5375-5383.
- Vardar, G., & Wood, T. K. (2005). Protein engineering of toluene-o-xylene monooxygenase from Pseudomonas stutzeri OX1 for enhanced chlorinated ethene degradation and o-xylene oxidation. Applied microbiology and biotechnology, 68(4), 510-517.
- Zheng, C. F., Wang, T. T., & Weiner, H. (1993). Cloning and Expression of the Full‐Length cDNAS Encoding Human Liver Class 1 and Class 2 Aldehyde Dehydrogenase. Alcoholism: Clinical and Experimental Research, 17(4), 828-831.
 "
"