Team:USTC CHINA/Project/Results
From 2013.igem.org
(Difference between revisions)
| Line 65: | Line 65: | ||
<p align="justify">As the construction and concentration of recombinant plasmid rely heavily on E.coli system in current molecule experiment protocols of Bacillus subtilis, Bacillus subtilis acts only as the secretory expression vector. Therefore, to verify the practicality of our locus and the transdermal function of recombinant transdermal protein on top of its original functions, we conducted basic experiments on E.coli to verify our assumptions. </p> | <p align="justify">As the construction and concentration of recombinant plasmid rely heavily on E.coli system in current molecule experiment protocols of Bacillus subtilis, Bacillus subtilis acts only as the secretory expression vector. Therefore, to verify the practicality of our locus and the transdermal function of recombinant transdermal protein on top of its original functions, we conducted basic experiments on E.coli to verify our assumptions. </p> | ||
<p align="justify">The following figure shows the stages of our basal experiments:</p> | <p align="justify">The following figure shows the stages of our basal experiments:</p> | ||
| - | <div align="center"><img src="https://static.igem.org/mediawiki/2013/ | + | <div align="center"><img src="https://static.igem.org/mediawiki/2013/d/d0/Basal_phase.png" width="300" height="400"/></div> |
</div> | </div> | ||
Revision as of 01:09, 28 September 2013
 "
"



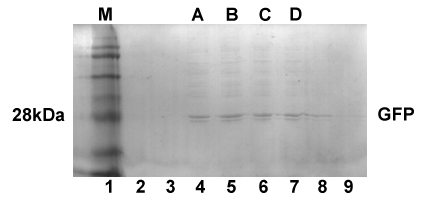 Fig1. SDS PAGE shows the molecule weight of TD1-GFP
Fig1. SDS PAGE shows the molecule weight of TD1-GFP





