Team:USTC CHINA/Project/Results
From 2013.igem.org
(Difference between revisions)
| (23 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
<link rel="stylesheet" type="text/css" href="https://2013.igem.org/Team:USTC_CHINA/main.css?action=raw&ctype=text/css" /> | <link rel="stylesheet" type="text/css" href="https://2013.igem.org/Team:USTC_CHINA/main.css?action=raw&ctype=text/css" /> | ||
<link rel="stylesheet" type="text/css" href="https://2013.igem.org/Team:USTC_CHINA/pro.css?action=raw&ctype=text/css" /> | <link rel="stylesheet" type="text/css" href="https://2013.igem.org/Team:USTC_CHINA/pro.css?action=raw&ctype=text/css" /> | ||
| + | |||
</head> | </head> | ||
<body background="https://static.igem.org/mediawiki/2013/6/62/2013ustc-china_Light_grey_bg.png"> | <body background="https://static.igem.org/mediawiki/2013/6/62/2013ustc-china_Light_grey_bg.png"> | ||
| Line 61: | Line 62: | ||
<div class="leftbar" align="left"> | <div class="leftbar" align="left"> | ||
<div class="basic-bar"> | <div class="basic-bar"> | ||
| - | <h1>Stage | + | <h1>Stage One:Basic Experiments</h1> |
<h2>Introduction</h2> | <h2>Introduction</h2> | ||
| - | <p align="justify">As the construction and concentration of recombinant plasmid rely heavily on E.coli system in current molecule experiment protocols of | + | <p align="justify">As the construction and concentration of recombinant plasmid rely heavily on <i>E.coli </i>system in current molecule experiment protocols of <i>B.subtilis</i>, <i>B.subtilis</i> acts only as the secretory expression vector. Therefore, to verify the practicality of our locus and the transdermal function of recombinant transdermal protein on top of its original functions, we conducted basic experiments on <i>E.coli</i> to verify our assumptions.</p> |
| - | <p align="justify">The following figure shows the stages of our | + | <p align="justify">The following figure shows the stages of our basic experiments:</p> |
<div align="center"><img src="https://static.igem.org/mediawiki/2013/d/d0/Basal_phase.png" width="300" height="400"/></div> | <div align="center"><img src="https://static.igem.org/mediawiki/2013/d/d0/Basal_phase.png" width="300" height="400"/></div> | ||
</div> | </div> | ||
| Line 71: | Line 72: | ||
<h2>Results</h2> | <h2>Results</h2> | ||
<h3>1. Verifying the Validity of Our Circuit by GFP</h3> | <h3>1. Verifying the Validity of Our Circuit by GFP</h3> | ||
| - | <p align="justify">E.coli BL21 proved that the standard transdermal locus does work with GFP.</p> | + | <p align="justify"><i>E.coli<i> BL21 proved that the standard transdermal locus does work with GFP.</p> |
| - | <img src="https://static.igem.org/mediawiki/ | + | <img src="https://static.igem.org/mediawiki/igem.org/7/77/2013ustc-china_gfp_xianlu.12.png" width="580" height="125"/> |
| - | <p align="justify">Using GFP to prove the validity of a newly designed circuit is a classical way to verify the expressing of this circuit. As expressions in E.coli involve neither secretory nor sequential problems, we hoped to verify the practicality of our locus by the expression of TD1-GFP. Thus we selected pET22b, which is a common recombinant vector for plasmid construction, as our recombinant vector and E.coli BL21 as engineered bacteria . We fused sequence TD1-GFP with T7 promoter from pET22b downstream and succeeded in expressing fusion protein TD1-GFP. </p> | + | <p align="justify">Using GFP to prove the validity of a newly designed circuit is a classical way to verify the expressing of this circuit. As expressions in <i>E.coli</i> involve neither secretory nor sequential problems, we hoped to verify the practicality of our locus by the expression of TD1-GFP. Thus we selected pET22b, which is a common recombinant vector for plasmid construction, as our recombinant vector and <i>E.coli</i> BL21 as engineered bacteria. We fused sequence TD1-GFP with T7 promoter from pET22b downstream and succeeded in expressing fusion protein TD1-GFP.</p> |
<div class="atfigure" align="center"><img src="https://static.igem.org/mediawiki/2013/d/d7/2013ustc-chinajiaotuTD1-GFP.jpg"></br></br> | <div class="atfigure" align="center"><img src="https://static.igem.org/mediawiki/2013/d/d7/2013ustc-chinajiaotuTD1-GFP.jpg"></br></br> | ||
Fig1. SDS PAGE shows the molecule weight of TD1-GFP</div> | Fig1. SDS PAGE shows the molecule weight of TD1-GFP</div> | ||
| Line 92: | Line 93: | ||
<div class="basic-bar"> | <div class="basic-bar"> | ||
<h3 style="font-size:24px;line-height:20px;">2. TD1 Fusion Protein Expression</h3> | <h3 style="font-size:24px;line-height:20px;">2. TD1 Fusion Protein Expression</h3> | ||
| - | <p align="justify">The expression of recombinant antigen and adjuvant in E.coli BL21.</p> | + | <p align="justify">The expression of recombinant antigen and adjuvant in <i>E.coli</i> BL21.</p> |
<img src="https://static.igem.org/mediawiki/2013/4/4d/2013ustc-china_genecircuit.png" width="580" height="120"/> | <img src="https://static.igem.org/mediawiki/2013/4/4d/2013ustc-china_genecircuit.png" width="580" height="120"/> | ||
| - | <p align="justify">The practicality of our | + | <p align="justify">The practicality of our circus afforded by TD1-GFP enabled our to express recombinant antigen TD1-HBsAg and recombinant adjuvant TD1-LTB successfully. So far our basic molecule experiments have ended with perfection.</p> |
<div style="float:left;width:200px;"> | <div style="float:left;width:200px;"> | ||
<img src="https://static.igem.org/mediawiki/2013/6/6f/2013ustc-chinasdsTD1-LTB.png" width="200" height="300"/> | <img src="https://static.igem.org/mediawiki/2013/6/6f/2013ustc-chinasdsTD1-LTB.png" width="200" height="300"/> | ||
| Line 109: | Line 110: | ||
<div class="basic-bar"> | <div class="basic-bar"> | ||
<h3 style="font-size:24px;line-height:20px;">3. Verifying the Antigenicity of TD1-Antigen by ELISA</h3> | <h3 style="font-size:24px;line-height:20px;">3. Verifying the Antigenicity of TD1-Antigen by ELISA</h3> | ||
| - | <p align="justify"> | + | <p align="justify"> The antigenicity of the TD1-antigen(fusion protein)strongly proved our theoretical basis. We have set 3 different negative control groups(GFP sample, HBsAg transdermal result, normal saline) and one positive group standard HBsAg sample.</p> |
| - | <img src="https://static.igem.org/mediawiki/2013/ | + | <img src="https://static.igem.org/mediawiki/2013/8/85/%E6%97%A0%E6%A0%87%E9%A2%98%2C%2C%2C%2C%2C.png" width="580" height="150"/> |
| - | <div class="atfigure" align="center" style="width:">Fig 4. Verifying the antigenicity of TD1-HBsAg by ELISA</div> | + | <div class="atfigure" align="center" style="width:">Fig 4. Verifying the antigenicity of TD1-HBsAg by ELISA.</div> |
</div> | </div> | ||
| Line 118: | Line 119: | ||
<p align="justify">TD1-HBsAg is able to penetrate the skin and keep its antigenicity: </p> | <p align="justify">TD1-HBsAg is able to penetrate the skin and keep its antigenicity: </p> | ||
<br> | <br> | ||
| - | <div | + | <div> |
| - | <img src="https://static.igem.org/mediawiki/2013/ | + | <img src="https://static.igem.org/mediawiki/2013/3/31/%E9%80%8F%E7%9A%AE%E8%A3%85%E7%BD%AE.png" width="580" height="300" /> |
<div class="atfigure" align="center" style="width:106px">Fig 5. Special transdermal device</div></div> | <div class="atfigure" align="center" style="width:106px">Fig 5. Special transdermal device</div></div> | ||
| - | <p align="justify">To prove that TD1-HBsAg is able to pass across the skin and keep its antigenicity, we utilized a | + | <p align="justify">To prove that TD1-HBsAg is able to pass across the skin and keep its antigenicity, we utilized a special device in the picture below, whose upper tube and lower tube can be separated by fresh skin peeled from mice, and all we were required to do was fastening the device, adding protein solution to the upper tube and extracting appropriate volume of liquid from the physiological saline in the lower tube to check the concentration of TD1-HBsAg which had crossed the skin during diverse periods.</p> |
<img src="https://static.igem.org/mediawiki/2013/a/a9/ELISA.jpg" width="580" height="380"/> | <img src="https://static.igem.org/mediawiki/2013/a/a9/ELISA.jpg" width="580" height="380"/> | ||
<div class="atfigure" align="center" style="width:">Fig 6. Check the concentration of TD1-HBsAg in the liquid under the skin during diverse periods with ELISA</div> | <div class="atfigure" align="center" style="width:">Fig 6. Check the concentration of TD1-HBsAg in the liquid under the skin during diverse periods with ELISA</div> | ||
</div> | </div> | ||
| - | <div style="float:right;font-size:26px;margin:20px auto 20px 0;"><a href="https://2013.igem.org/Team:USTC_CHINA/Project/ | + | <div style="float:right;font-size:26px;margin:20px auto 20px 0;"><a href="https://2013.igem.org/Team:USTC_CHINA/Project/AdvancingWork">Advancing Work >>></a></div> |
</div> | </div> | ||
<div class="rightbar"> | <div class="rightbar"> | ||
| Line 136: | Line 137: | ||
<div id="t1"><a class="active" href="https://2013.igem.org/Team:USTC_CHINA/Project/Results">Results</a></div> | <div id="t1"><a class="active" href="https://2013.igem.org/Team:USTC_CHINA/Project/Results">Results</a></div> | ||
<div id="t2"><a class="active" href="https://2013.igem.org/Team:USTC_CHINA/Project/Results">Basic Experiment</a></div> | <div id="t2"><a class="active" href="https://2013.igem.org/Team:USTC_CHINA/Project/Results">Basic Experiment</a></div> | ||
| - | <div id="t2"><a href="https://2013.igem.org/Team:USTC_CHINA/Project/ | + | <div id="t2"><a href="https://2013.igem.org/Team:USTC_CHINA/Project/AdvancingWork">Advancing Work</a></div> |
| + | <div id="t2"><a href="https://2013.igem.org/Team:USTC_CHINA/Project/FutureWork">Future Work</a></div> | ||
<div id="t1"><a href="https://2013.igem.org/Team:USTC_CHINA/Parts">Parts</a></div> | <div id="t1"><a href="https://2013.igem.org/Team:USTC_CHINA/Parts">Parts</a></div> | ||
Latest revision as of 12:30, 28 October 2013
 "
"



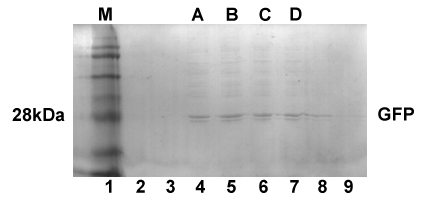 Fig1. SDS PAGE shows the molecule weight of TD1-GFP
Fig1. SDS PAGE shows the molecule weight of TD1-GFP






