|
|
| (8 intermediate revisions not shown) |
| Line 30: |
Line 30: |
| | | | |
| | <font color="#333399" size="3" font face="Calibri"> | | <font color="#333399" size="3" font face="Calibri"> |
| - | | + | **still needs work |
| - | ADD large Phage project overview
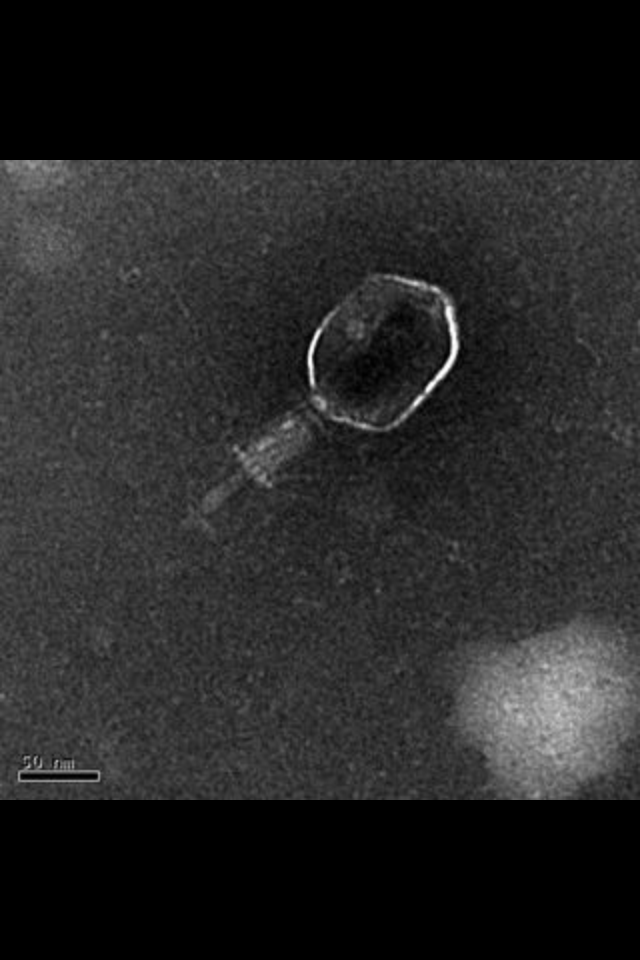
| + | :The goal of the Large Phage Project is to make a mutant phage that has a capsid much larger than the normal ~200nm T4 capsid. Our intent is to create a protocol where large phage with stable phenotypes will form making it possible to "pick a size" when using phage as a delivery system. The impact of these large phage could mean the difference in making a drug treatment effective and efficient. |
| - | [[File:Example.gif]] | + | :Through the use of our mutagen 5'bromodeoxyuridine, a good mutagenesis protocol and the use of a sensitive cesium chloride gradient. We were able to mutagenize and isolate giant mutant t4 phage. |
| - | | + | [[File:EMpic-1.jpg|400px]] |
| | | | |
| | | | |
| Line 43: |
Line 43: |
| | {{TeamBYUProvoFooter}} | | {{TeamBYUProvoFooter}} |
| | | | |
| - | ==March==
| |
| - | ===3/15/13===
| |
| - |
| |
| - | ===3/18/13===
| |
| - |
| |
| - | ===3/20/13===
| |
| - |
| |
| - | ===3/22/13===
| |
| - |
| |
| - | ===3/25/13===
| |
| - |
| |
| - | ===3/27/13===
| |
| - |
| |
| - | ===3/29/13===
| |
| - |
| |
| - | ==April==
| |
| - | ===4/1/13===
| |
| - |
| |
| - | ===4/4/13===
| |
| - |
| |
| - | ===4/5/13===
| |
| - |
| |
| - | ===4/8/13===
| |
| - |
| |
| - | ===4/10/13===
| |
| - |
| |
| - | ===4/12/13===
| |
| - |
| |
| - | ===4/15/13===
| |
| - |
| |
| - | ==May==
| |
| - | ===5/1/13===
| |
| - |
| |
| - | ===5/3/13===
| |
| - |
| |
| - | ===5/6/13===
| |
| - |
| |
| - | ===5/8/13===
| |
| - |
| |
| - | ===5/10/13===
| |
| - |
| |
| - | ===5/13/13===
| |
| - |
| |
| - | ===5/15/13===
| |
| - |
| |
| - | ===5/17/13===
| |
| - |
| |
| - | ===5/20/13===
| |
| - | Today we need to run a dilution series to test the titer of our mutated phage stock.
| |
| - | We also need to start selecting for small plaques and learning how to pick them and titer them out.
| |
| - | We also should run a UV test on the mutated phage stock compared to the normal stock.
| |
| - |
| |
| - | We picked one plaque off of the 180 sec UV plate sample. We suspended it in 1 mL of broth, and then UV-ed 20 uL samples at 45 second intervals. The number of plaques decreased the longer the samples sat under UV light. The samples were irradiated from 0 sec to 4 min 30 sec.
| |
| - |
| |
| - | As a control, we diluted the T4Do stock to 10^-6 and tested 20 uL at 45 sec intervals (up to 6 min) under UV light.
| |
| - |
| |
| - | Also, we diluted the T4 mutagenized stock to 10^-6 and tested 20 uL at 45 sec intervals (up to 4 min 30 sec) under UV light.
| |
| - |
| |
| - | UV tests were done by placing 20 uL spots on parafilm and placed in a BSL-2 hood with the UV light turned on.
| |
| - |
| |
| - | ===5/22/13===
| |
| - | Results from 5/20/13 -
| |
| - | We left the plates in the incubator for 48 hours, which caused contamination on many plates to grow.
| |
| - |
| |
| - | When checked at 24 hours, the T4 mutagenized stock had a web plate at 10^-3 and less than 5 plaques at 10^-6. This experiment will need to be redone from 10^0 down through 10^-6. The whole mutagenesis may need to be redone if this only represents a dilution of our titer when we were trying to grow it in liquid culture.
| |
| - | (We infected with ___ mL of phage at ___ titer in ____ vol of resuspended bacteria.)
| |
| - |
| |
| - | Under UV light, the T4Do stock (diluted to 10^-6) has 19 plaques after being irradiated for six minutes (down from almost cleared at 0 min).
| |
| - | Under UV light, the 180 sec UV plate spot (diluted in 1 mL) has a few hundred plaques on it, but the amount dropped significantly at 4 min 30 sec from when it was UV-ed for 0 min.
| |
| - |
| |
| - | We will re-titer the T4-Mut stock so we can learn whether it was diluted or whether an infection worked.
| |
| - | We will also re-run the UV test comparing the T4-Mut (10^-3) with the T4-Do stock (at 10^-6) for survivability. The mutagenized phage should survive better.
| |
| - |
| |
| - | Since the loss of plaques seemed to level off for the T4Do stock at 5:15 (23 plaques) and 6 min (19 plaques), we will test a 7 min and 8 min timepoint to see if it stays level, suggesting these phage have multiple genomes and are severely mutated.
| |
| - | [[File:Apple.jpeg|600px|thumb|center|T4 mutant 0 and 1.5 minutes under UV light.]]
| |
| - | [[File:Blackberry1.jpeg|600px|thumb|center|T4 mutant 3 and 4.5 minutes under UV light.]]
| |
| - | [[File:Cranberry.jpeg|600px|thumb|center|T4 mutant 6 minutes under UV light.]]
| |
| - | [[File:Dingleberry.jpeg|600px|thumb|center|T4 mutant 7.5 minutes under UV light.]]
| |
| - | [[File:Eggplant.jpeg|600px|thumb|center|T4 mutant 9 minutes under UV light.]]
| |
| - | [[File:Eggplant.jpeg|600px|thumb|center|T4 mutant 0 and 1.5 minutes under UV light.]]
| |
| - |
| |
| - | ===5/24/13===
| |
| - |
| |
| - | ===5/27/13===
| |
| - |
| |
| - | ===5/29/13===
| |
| - | Today we wanted to see if we can transfer our current experiment from using E. coli W3110 as the host to E. coli B as the host. We set up multiple experiments.
| |
| - | 1. We spot tested the following phage samples on W3110 and B: T1, T2, T3, T4Do stock, T4 mutated stock, T4-UV one-plaque plate harvest, T4-UV-mutated one-plaque plate harvest.
| |
| - | 2. We did a dilution series on the T1 stock to see how concentrated it is. We will need to know the PFUs in order to grow a successful liquid culture and mass produce it for our large phage amplification procedure.
| |
| - | 3. We also infected two samples of E. coli B, each with 10 uL of 10^-6 T4Do to observe plaque formation.
| |
| - |
| |
| - | ===5/31/13===
| |
| - |
| |
| - | ==June==
| |
| - | ===6/3/13===
| |
| - |
| |
| - | ===6/5/13===
| |
| - |
| |
| - | ===6/7/13===
| |
| - |
| |
| - | ===6/10/13===
| |
| - |
| |
| - | ===6/12/13===
| |
| - |
| |
| - | ===6/14/13===
| |
| - |
| |
| - | ===6/17/13===
| |
| - |
| |
| - | ===6/19/13===
| |
| - |
| |
| - | ===6/21/13===
| |
| - |
| |
| - | ===6/24/13===
| |
| - |
| |
| - | ===6/26/13===
| |
| - |
| |
| - | ===6/28/13===
| |
| - |
| |
| - | ==July==
| |
| - | ===7/1/13===
| |
| - |
| |
| - | ===7/3/13===
| |
| - |
| |
| - | ===7/5/13===
| |
| - |
| |
| - | ===7/8/13===
| |
| - |
| |
| - | ===7/10/13===
| |
| - |
| |
| - | ===7/12/13===
| |
| - |
| |
| - | ===7/15/13===
| |
| - |
| |
| - | ===7/17/13===
| |
| - |
| |
| - | ===7/19/13===
| |
| - |
| |
| - | ===7/22/13===
| |
| - |
| |
| - | ===7/24/13===
| |
| - |
| |
| - | ===7/26/13===
| |
| - |
| |
| - | ===7/29/13===
| |
| - |
| |
| - | ===7/31/13===
| |
| - |
| |
| - | ==August==
| |
| - | ===8/2/13===
| |
| - |
| |
| - | ===8/5/13===
| |
| - |
| |
| - | ===8/7/13===
| |
| - |
| |
| - | ===8/9/13===
| |
| - |
| |
| - | ===8/12/13===
| |
| - |
| |
| - | ===8/14/13===
| |
| - |
| |
| - | ===8/16/13===
| |
| - |
| |
| - | ===8/19/13===
| |
| - |
| |
| - | ===8/21/13===
| |
| - |
| |
| - | ===8/23/13===
| |
| - |
| |
| - | ===8/26/13===
| |
| - |
| |
| - | ===8/28/13===
| |
| | | | |
| - | ===8/30/13===
| |
| | | | |
| | ==September== | | ==September== |
 "
"