Team:DTU-Denmark/Notebook/11 July 2013
From 2013.igem.org
(→Gel analyses of Nir operon, yesterday's PCR product) |
|||
| (22 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | =208= | + | {{:Team:DTU-Denmark/Templates/StartPage|11 July 2013}} |
| + | Navigate to the [[Team:DTU-Denmark/Notebook/10_July_2013|Previous]] or the [[Team:DTU-Denmark/Notebook/12_July_2013|Next]] Entry | ||
| + | =Lab 208= | ||
| + | <hr/> | ||
==Main purpose== | ==Main purpose== | ||
| + | <hr/> | ||
*PCR with USER primers to [[Team:DTU-Denmark/Notebook/11_July_2013#Combine_Nir_operon_with_pZA21|combine Nir operon with pZA21]] | *PCR with USER primers to [[Team:DTU-Denmark/Notebook/11_July_2013#Combine_Nir_operon_with_pZA21|combine Nir operon with pZA21]] | ||
| Line 6: | Line 10: | ||
==Who was in the lab== | ==Who was in the lab== | ||
| - | Ariadni,Henrike,Julia,Jakob | + | <hr/> |
| + | Ariadni, Henrike, Julia, Jakob, Gosia | ||
==Procedure== | ==Procedure== | ||
| - | + | <hr/> | |
===Combine Nir operon with pZA21=== | ===Combine Nir operon with pZA21=== | ||
| Line 17: | Line 22: | ||
triplets of each of the two Nir Operon extracts that we have made yesterday, including one negative control: | triplets of each of the two Nir Operon extracts that we have made yesterday, including one negative control: | ||
| - | *Nir | + | *Nir 1(a, b, c) |
| - | *Nir | + | *Nir 2(a, b, c) |
Using the following PCR program: | Using the following PCR program: | ||
| Line 40: | Line 45: | ||
===PCR for promoter test=== | ===PCR for promoter test=== | ||
| - | We want to test the inducible | + | We want to test the inducible AraBAD promoter using plasmid pZA21 without its native promoter and RFP gene. |
We performed 3 different PCR reactions using user primers. | We performed 3 different PCR reactions using user primers. | ||
| + | Each PCR reaction was performed in triplicate. | ||
| + | |||
Samples are named: | Samples are named: | ||
*1,2,3 -> pZA21 with no native promoter - Primers 13a and 13b, template pZA21 miniprep | *1,2,3 -> pZA21 with no native promoter - Primers 13a and 13b, template pZA21 miniprep | ||
*4,5,6 -> RFP - Primers 14a and 14b, template RFP in pZE21 miniprep | *4,5,6 -> RFP - Primers 14a and 14b, template RFP in pZE21 miniprep | ||
| - | *7,8,9 -> | + | *7,8,9 -> AraBAD promoter - Primers 12a and 12b, template biobrick K808000 |
Settings for PCR of 1,2,3 | Settings for PCR of 1,2,3 | ||
| - | {| class="wikitable | + | {| class="wikitable" style="text-align: right" |
! Temperature (<sup>o</sup>C)!! Time (min)!! Rounds | ! Temperature (<sup>o</sup>C)!! Time (min)!! Rounds | ||
|- | |- | ||
| Line 65: | Line 72: | ||
Settings for PCR of 4-9 | Settings for PCR of 4-9 | ||
| - | {| class="wikitable | + | {| class="wikitable" style="text-align: right" |
! Temperature (<sup>o</sup>C)!! Time (min)!! Rounds | ! Temperature (<sup>o</sup>C)!! Time (min)!! Rounds | ||
|- | |- | ||
| Line 81: | Line 88: | ||
|} | |} | ||
| - | ===Gel analyses of Nir operon, PCR product from 10-07-2013=== | + | ===Gel analyses of Nir operon, PCR product from [[Team:DTU-Denmark/Notebook/10_July_2013#Extraction_PCR|10-07-2013]]=== |
We run 0,8% agarose gel with 9 samples. | We run 0,8% agarose gel with 9 samples. | ||
| + | |||
| + | ===PCR purification=== | ||
| + | PCR fragments for the Nir operon from todays PCR. | ||
| + | |||
| + | ==Result== | ||
| + | <hr/> | ||
| + | 0.8 % Agorase Gel to test yesterday's PCR of the Nir operon with different extension times | ||
| + | |||
| + | Wells | ||
| + | *1: Nir extension time 2:00 | ||
| + | *2: Nir extension time 2:00 | ||
| + | *3: Nir extension time 2:00 | ||
| + | *4: Nir extension time 3:00 | ||
| + | *5: 1kb ladder | ||
| + | *6: Nir extension time 3:00 | ||
| + | *7: Nir extension time 3:00 | ||
| + | *8: Nir extension time 4:00 | ||
| + | *9: Nir extension time 4:00 | ||
| + | *10: Nir extension time 4:00 | ||
| + | |||
| + | [[File:2013-07-11_Nir_PCr_results.jpg|600px]] | ||
| + | |||
| + | ==Conclusion== | ||
| + | <hr/> | ||
| + | We have now tried PCR of Nir more than one time and same result appear. This must then be the true chromosomal fragment but with some additional sequence in the middle. We will try to do another PCR with USER primers tomorrow to see if we then get same fragments or shorter ones. | ||
| + | |||
| + | |||
| + | Navigate to the [[Team:DTU-Denmark/Notebook/10_July_2013|Previous]] or the [[Team:DTU-Denmark/Notebook/12_July_2013|Next]] Entry | ||
| + | |||
| + | {{:Team:DTU-Denmark/Templates/EndPage}} | ||
Latest revision as of 20:40, 16 September 2013
11 July 2013
Contents |
Lab 208
Main purpose
- PCR with USER primers to combine Nir operon with pZA21
- PCR with USER primer to test the activity of the promoter AraB.
Who was in the lab
Ariadni, Henrike, Julia, Jakob, Gosia
Procedure
Combine Nir operon with pZA21
Following Mathildes Protocol for DpnI digestion of our linear pZA21 w. Uracil incerts
Mastermix for 7 PCR tubes, Using the mastermix protocol we got from Mathilde.
triplets of each of the two Nir Operon extracts that we have made yesterday, including one negative control:
- Nir 1(a, b, c)
- Nir 2(a, b, c)
Using the following PCR program:
| time | Temp C | |
|---|---|---|
| Denaturing | 2 min | 98 |
| start of loop | 10 sec | 98 |
| annealing | 30 sec | 60 |
| elongation | 9 min | 72 |
| goto start of loop 35 times | ||
| hold | 10 |
PCR for promoter test
We want to test the inducible AraBAD promoter using plasmid pZA21 without its native promoter and RFP gene. We performed 3 different PCR reactions using user primers. Each PCR reaction was performed in triplicate.
Samples are named:
- 1,2,3 -> pZA21 with no native promoter - Primers 13a and 13b, template pZA21 miniprep
- 4,5,6 -> RFP - Primers 14a and 14b, template RFP in pZE21 miniprep
- 7,8,9 -> AraBAD promoter - Primers 12a and 12b, template biobrick K808000
Settings for PCR of 1,2,3
| Temperature (oC) | Time (min) | Rounds |
|---|---|---|
| 98 | 2:00 | 1 |
| 98 | 0:20 | 35 |
| 58 | 0:45 | 35 |
| 72 | 2:00 | 35 |
| 72 | 5:00 | 1 |
| 10 | ∞ | - |
Settings for PCR of 4-9
| Temperature (oC) | Time (min) | Rounds |
|---|---|---|
| 98 | 2:00 | 1 |
| 98 | 0:20 | 35 |
| 61 | 0:45 | 35 |
| 72 | 1:00 | 35 |
| 72 | 5:00 | 1 |
| 10 | ∞ | - |
Gel analyses of Nir operon, PCR product from 10-07-2013
We run 0,8% agarose gel with 9 samples.
PCR purification
PCR fragments for the Nir operon from todays PCR.
Result
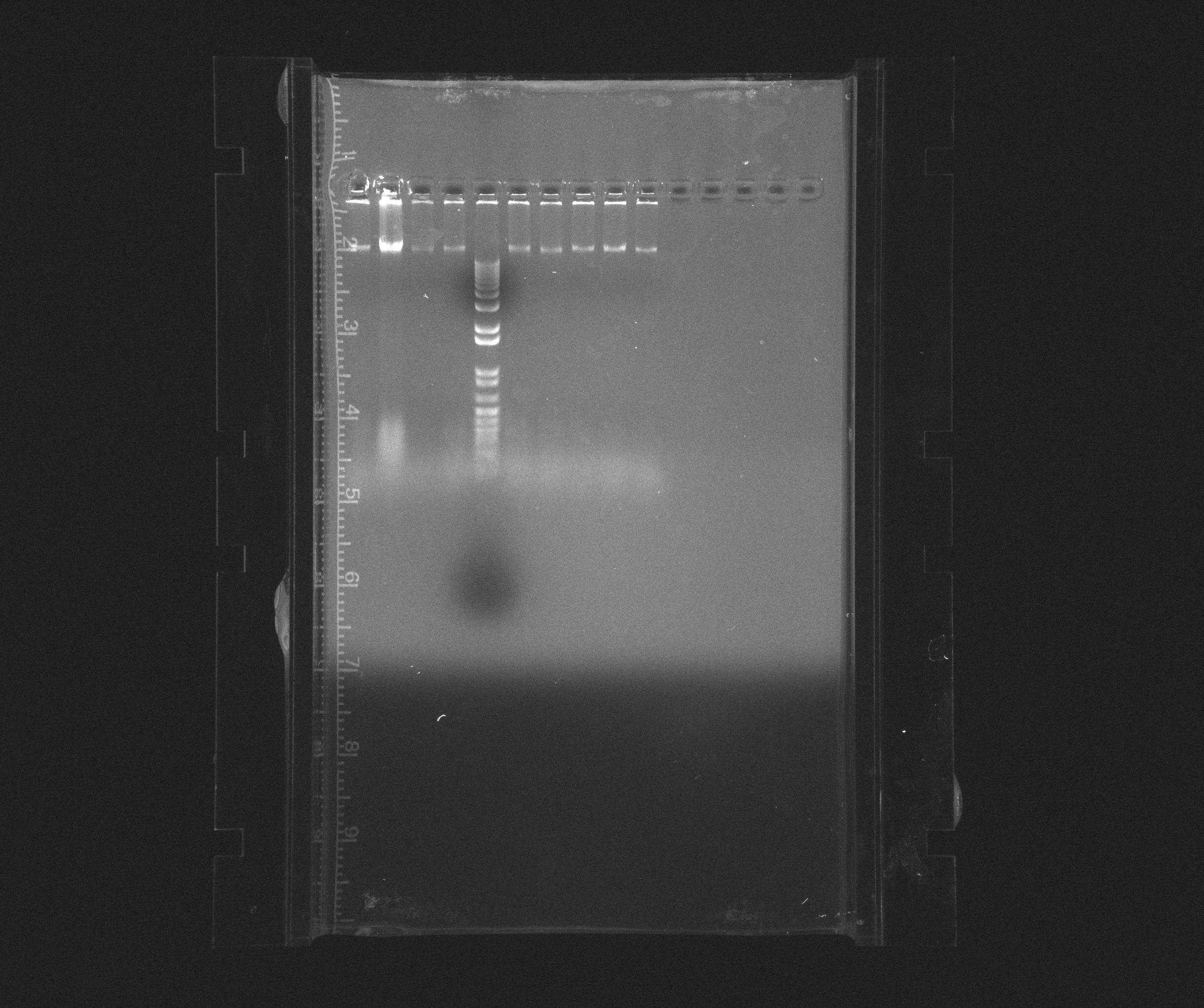
0.8 % Agorase Gel to test yesterday's PCR of the Nir operon with different extension times
Wells
- 1: Nir extension time 2:00
- 2: Nir extension time 2:00
- 3: Nir extension time 2:00
- 4: Nir extension time 3:00
- 5: 1kb ladder
- 6: Nir extension time 3:00
- 7: Nir extension time 3:00
- 8: Nir extension time 4:00
- 9: Nir extension time 4:00
- 10: Nir extension time 4:00
Conclusion
We have now tried PCR of Nir more than one time and same result appear. This must then be the true chromosomal fragment but with some additional sequence in the middle. We will try to do another PCR with USER primers tomorrow to see if we then get same fragments or shorter ones.
Navigate to the Previous or the Next Entry
 "
"