Template:Kyoto/Notebook/Sep 3
From 2013.igem.org
(Difference between revisions)
(→Electrophoresis) |
(→Gel Extraction) |
||
| Line 352: | Line 352: | ||
|1 ||100bp ladder || | |1 ||100bp ladder || | ||
|- | |- | ||
| - | |3 ||pT181 | + | |3 ||pT181 attenuator||EcoRI&SpeI |
|- | |- | ||
| - | |4 ||pT181 | + | |4 ||pT181 attenuator||EcoRI&SpeI |
|- | |- | ||
| - | |6 ||pT181 | + | |6 ||pT181 attenuator||XbaI&PstI |
|- | |- | ||
| - | |7 ||pT181 | + | |7 ||pT181 attenuator||XbaI&PstI |
|- | |- | ||
| - | |9 ||pT181 antisense|| | + | |9 ||pT181 antisense||EcoRI&SpeI |
|- | |- | ||
| - | |10 ||pT181 antisense|| | + | |10 ||pT181 antisense||EcoRI&SpeI |
|} | |} | ||
[[File:Igku Sep3 Electrophoresis(N4_1).jpg]]<br> | [[File:Igku Sep3 Electrophoresis(N4_1).jpg]]<br> | ||
| Line 370: | Line 370: | ||
|1 ||100bp ladder || | |1 ||100bp ladder || | ||
|- | |- | ||
| - | |2 ||pT181 antisense|| | + | |2 ||pT181 antisense||XbaI&PstI |
|- | |- | ||
| - | |3 ||pT181 antisense|| | + | |3 ||pT181 antisense||XbaI&PstI |
|- | |- | ||
| - | |5 ||apt12_1R|| | + | |5 ||apt12_1R||EcoRI&SpeI |
|- | |- | ||
| - | |6 ||apt12_1R|| | + | |6 ||apt12_1R||EcoRI&SpeI |
|} | |} | ||
[[File:Igku Sep3 Electrophoresis(N4_2).jpg]]<br> | [[File:Igku Sep3 Electrophoresis(N4_2).jpg]]<br> | ||
| Line 384: | Line 384: | ||
|1 ||100bp ladder || | |1 ||100bp ladder || | ||
|- | |- | ||
| - | |2 ||DT|| | + | |2 ||DT||EcoRI&XbaI |
|- | |- | ||
| - | |3 ||DT|| | + | |3 ||DT||EcoRI&XbaI |
|- | |- | ||
| - | |5 ||RBS-lysis1| | + | |5 ||RBS-lysis1|EcoRI&SpeI |
|- | |- | ||
| - | |6 ||RBS-lysis1|| | + | |6 ||RBS-lysis1||EcoRI&SpeI |
|} | |} | ||
[[File:Igku Sep3 Electrophoresis(N5_3).jpg]]<br> | [[File:Igku Sep3 Electrophoresis(N5_3).jpg]]<br> | ||
| Line 398: | Line 398: | ||
|1 ||100bp ladder || | |1 ||100bp ladder || | ||
|- | |- | ||
| - | |2 ||RBS-lysis2|| | + | |2 ||RBS-lysis2||EcoRI&SpeI |
|- | |- | ||
| - | |3 ||RBS-lysis2|| | + | |3 ||RBS-lysis2||EcoRI&SpeI |
|- | |- | ||
| - | |5 ||RBS-lysis3|| | + | |5 ||RBS-lysis3||EcoRI&SpeI |
|- | |- | ||
| - | |6 ||RBS-lysis3|| | + | |6 ||RBS-lysis3||EcoRI&SpeI |
|} | |} | ||
[[File:Igku Sep3 Electrophoresis(N5_4).jpg]]<br> | [[File:Igku Sep3 Electrophoresis(N5_4).jpg]]<br> | ||
| Line 410: | Line 410: | ||
!Name||concentration[µg/mL]||260/280||260/230 | !Name||concentration[µg/mL]||260/280||260/230 | ||
|- | |- | ||
| - | |pT181 | + | |pT181 attenuator(EcoRI&SpeI)||10.0 ||1.86 ||0.39 |
|- | |- | ||
| - | |pT181 | + | |pT181 attenuator(XbaI&PstI) ||11.7 ||1.65 ||0.36 |
|- | |- | ||
| - | |pT181 antisense( | + | |pT181 antisense(EcoRI&SpeI) ||4.2 ||1.95 ||0.25 |
|- | |- | ||
| - | |pT181 antisense( | + | |pT181 antisense(XbaI&PstI)||6.6 ||1.78 ||0.33 |
|- | |- | ||
| - | |tRNA 12_1R apt( | + | |tRNA 12_1R apt(EcoRI&SpeI)||5.7 ||1.73 ||0.29 |
|- | |- | ||
| - | |DT( | + | |DT(EcoRI&XbaI) ||16.6 ||1.99 ||0.76 |
|- | |- | ||
| - | |RBS-lysis1( | + | |RBS-lysis1(EcoRI&SpeI)||8.8 ||1.65 ||0.32 |
|- | |- | ||
| - | |RBS-lysis2( | + | |RBS-lysis2(EcoRI&SpeI)||13.4 ||1.61 ||0.48 |
|- | |- | ||
| - | |RBS-lysis3( | + | |RBS-lysis3(EcoRI&SpeI)||10.2 ||2.19 ||0.51 |
|} | |} | ||
</div> | </div> | ||
Revision as of 16:10, 25 September 2013
Contents |
Sep 3
Electrophoresis
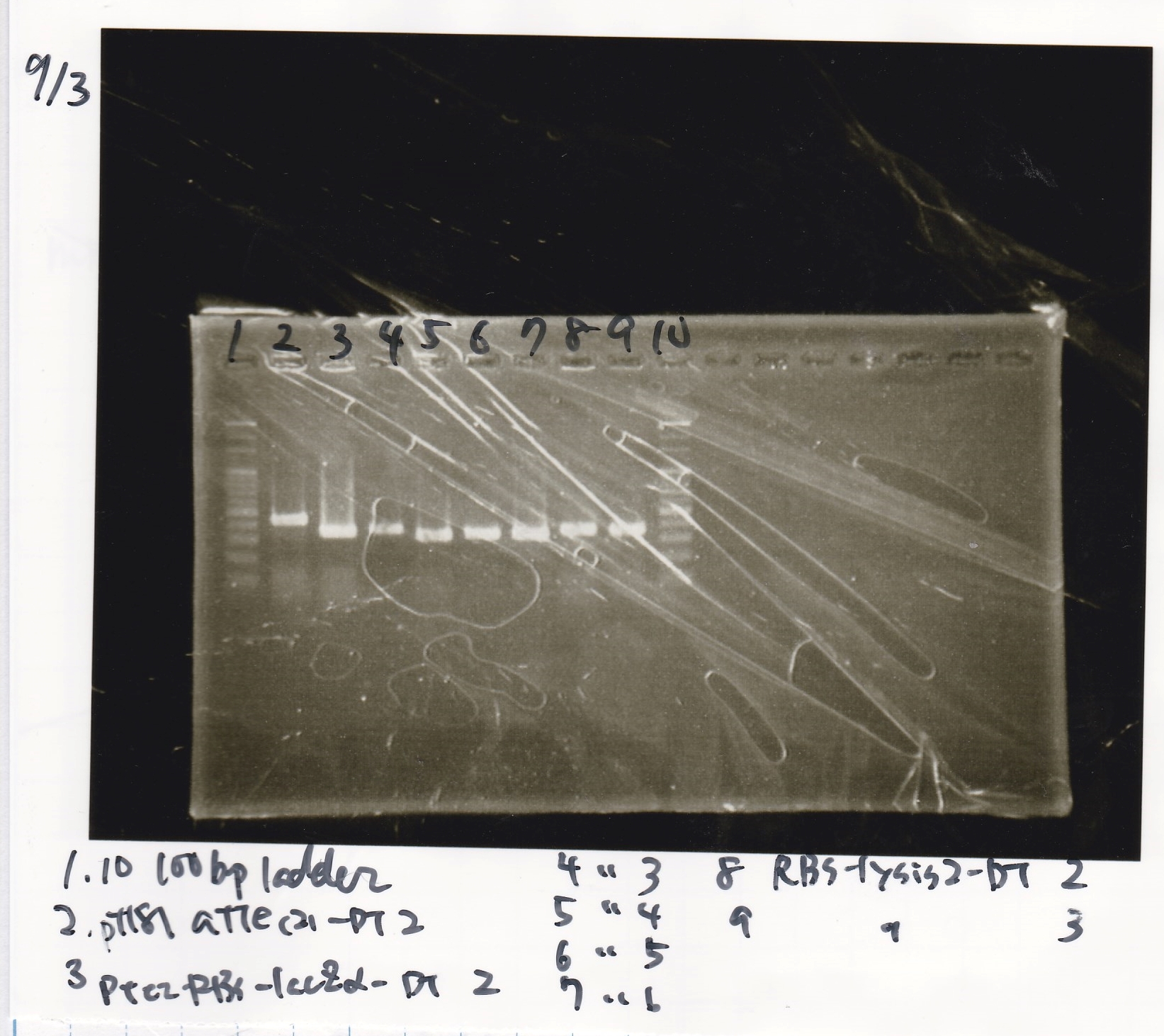
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | pT181 attenuator(2)-DT2(738) |
| 3 | Ptet-RBS-lacZα-DT-2(756) |
| 4 | Ptet-RBS-lacZα-DT-3 |
| 5 | Ptet-RBS-lacZα-DT-4 |
| 6 | Ptet-RBS-lacZα-DT-5 |
| 7 | Ptet-RBS-lacZα-DT-6 |
| 8 | RBS-lysis2-DT-2(985) |
| 9 | RBS-lysis2-DT-3 |
| 10 | 100bp ladder |
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 9/2 14:00 pT181 attenuator | 418.4 | 1.82 | 1.80 |
| 9/2 14:00 pT181 antisense | 362.2 | 1.92 | 1.96 |
| 9/2 14:00 Spinach | 438.3 | 1.86 | 1.99 |
| 9/2 14:00 tetR-apt 12_1R | 567.5 | 1.90 | 1.99 |
| 9/2 21:00 pT181 attenuator | 320.9 | 1.90 | 2.15 |
| 9/2 21:00 Spinach | 457.0 | 1.88 | 2.24 |
Liquid Culture
| Sample | medium |
|---|---|
| Spinach-DT(9/2 master plate) | plusgrow(CP)4ml |
Restriction Enzyme Digestion
| DNA | EcoR1 | Spe1 | Xba1 | Pst1 | 10xBuffer B(EcoR1&Spe1) | 10xBuffer D(Xba1&Pst1) | 100xBSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|---|
| 9/3 pT181attenuator 14:00 418.4ng/µL | 4.8µL | 1.0µL | 1.0µL | 0µL | 0µL | 3µL | 0µL | 0.3µL | 19.9µL | 30µL |
| 9/3 pT181attenuator 14:00 418.4ng/µL | 0.2µL | 0µL | 0µL | 0µL | 0µL | 1µL | 0µL | 0.1µL | 8.7µL | 10µL |
| 9/3 pT181attenuator 14:00 418.4ng/µL | 4.8µL | 0µL | 0µL | 1.0µL | 1.0µL | 0µL | 3µL | 0.3µL | 19.9µL | 30µL |
| 9/3 pT181attenuator 14:00 418.4ng/µL | 0.2µL | 0µL | 0µL | 0µL | 0µL | 0µL | 1µL | 0.1µL | 8.7µL | 10µL |
| DNA | EcoR1 | Spe1 | Xba1 | Pst1 | 10xBuffer B(EcoR1&Spe1) | 10xBuffer D(Xba1&Pst1) | 100xBSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|---|
| 9/3 pT181antisense 14:00 362.2ng/µL | 5.5µL | 1.0µL | 1.0µL | 0µL | 0µL | 3µL | 0µL | 0.3µL | 19.2µL | 30µL |
| 9/3 pT181attenuator 14:00 418.4ng/µL | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 0µL | 0.1µL | 8.6µL | 10µL |
| 9/3 pT181attenuator 14:00 418.4ng/µL | 5.5µL | 0µL | 0µL | 1.0µL | 1.0µL | 0µL | 3µL | 0.3µL | 19.2µL | 30µL |
| 9/3 pT181attenuator 14:00 418.4ng/µL | 0.3µL | 0µL | 0µL | 0µL | 0µL | 0µL | 1µL | 0.1µL | 8.6µL | 10µL |
| DNA | EcoR1 | Spe1 | 10xBuffer B | 100xBSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 9/3 apt 12_1R 14:00 507.5ng/µL | 3.9µL | 1.0µL | 1.0µL | 3µL | 0.3µL | 20.8µL | 30µL |
| 9/3 apt 12_1R 14:00 507.5ng/µL | 0.2µL | 0µL | 0µL | 1µL | 0.1µL | 8.7µL | 10µL |
| DNA | EcoR1 | Xba1 | 10xBuffer D | 100xBSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 8/29 DT 166.2ng/µL | 12µL | 1.0µL | 1.0µL | 3µL | 0.3µL | 12.7µL | 30µL |
| 8/29 DT 166.2ng/µL | 0.6µL | 0µL | 0µL | 1µL | 0.1µL | 8.3µL | 10µL |
| DNA | EcoR1 | Spe1 | 10xBuffer B | 100xBSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 8/22 RBS-lysis1(2) 96ng/µL | 21µL | 1.0µL | 1.0µL | 3µL | 0.3µL | 3.7µL | 30µL |
| 8/22 RBS-lysis1(2) 96ng/µL | 1.0µL | 0µL | 0µL | 1µL | 0.1µL | 7.9µL | 10µL |
| DNA | EcoR1 | Spe1 | 10xBuffer B | 100xBSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 8/30 RBS-lysis2 220ng/µL | 9.1µL | 1.0µL | 1.0µL | 3µL | 0.3µL | 15.6µL | 30µL |
| 8/30 RBS-lysis2 220ng/µL | 0.5µL | 0µL | 0µL | 1µL | 0.1µL | 8.4µL | 10µL |
| DNA | EcoR1 | Spe1 | 10xBuffer B | 100xBSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 8/20 RBS-lysis3(1) 282ng/µL | 7.1µL | 1.0µL | 1.0µL | 3µL | 0.3µL | 17.6µL | 30µL |
| 8/20 RBS-lysis3(1) 282ng/µL | 0.4µL | 0µL | 0µL | 1µL | 0.1µL | 8.5µL | 10µL |
PCR
| Plac(1) plasmid 153.7ng/µL | 10x buffer KOD plus-ver.2 | 2mM dNTPs | 25mM MgSO4 | primer (KaiA_BsaI_Mut_fwd) | primer (KaiA_BsaI_Mut_rer) | KOD plus-ver.2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.13 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 16.37 | 25 |
| KaiB (plasmid) 20.2ng/µL | 10x buffer KOD plus-ver.2 | 2mM dNTPs | 25mM MgSO4 | primer (GG2_RBS_KaiB_fwd) | primer (KaiB_GG3_rer) | KOD plus-ver.2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 15.5 | 25 |
| KaiC (plasmid) 4.4ng/µL | 10x buffer KOD plus-ver.2 | 2mM dNTPs | 25mM MgSO4 | primer | primer | KOD plus-ver.2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 15.5 | 25 |
| DT-1 (plasmid) 153.7ng/µL | 10x buffer KOD plus-ver.2 | 2mM dNTPs | 25mM MgSO4 | primer | primer | KOD plus-ver.2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.1 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 16.4 | 25 |
| PKaiBC(PCR product) 16.3ng/µL | 10x buffer KOD plus-ver.2 | 2mM dNTPs | 25mM MgSO4 | primer | primer | KOD plus-ver.2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.7 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 15.8 | 25 |
| SasA(PCR product) 21.6ng/µL | 10x buffer KOD plus-ver.2 | 2mM dNTPs | 25mM MgSO4 | primer | primer | KOD plus-ver.2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 16 | 25 |
| RpaA(PCR product) 15.4ng/µL | 10x buffer KOD plus-ver.2 | 2mM dNTPs | 25mM MgSO4 | primer | primer | KOD plus-ver.2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.6 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 15.9 | 25 |
| RpaB(PCR product) 26.7ng/µL | 10x buffer KOD plus-ver.2 | 2mM dNTPs | 25mM MgSO4 | primer | primer | KOD plus-ver.2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.4 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 16.1 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 68°C | -- | |
| 5min | 10sec | 30sec | 30 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 68°C | -- | |
| 5min | 10sec | 30sec | 30 |
Mutatation PCR
| KaiABC(12.6pg/µL) | Primer(Kai_BsaIMut_fwd) | Primer(Kai_BsaIMut_rer) | Prime STAR Max Premix | MilliQ | total |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 25 | 22 | 50 |
| Primer(Kai_BsaIMut_fwd) | Primer(Kai_BsaIMut_rer) | Prime STAR Max Premix | MilliQ | total | |
|---|---|---|---|---|---|
| 1 | 1 | 25 | 23 | 50 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 65°C | 72°C | -- |
| 5min | 10sec | 15sec | 40sec | 30 |
Colony PCR
| Sample | base pair |
|---|---|
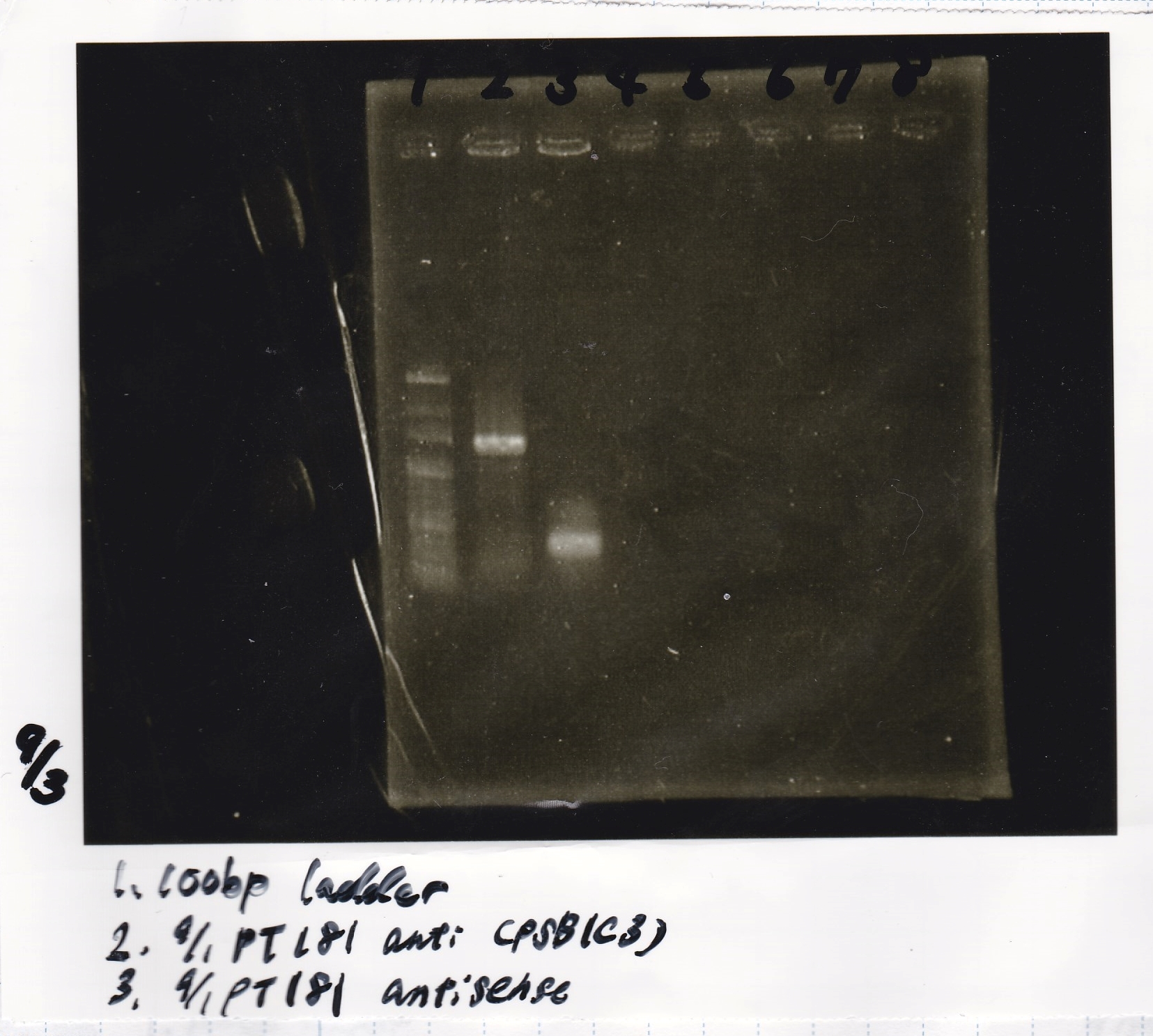
| 9/1 pT181antisense(pSB1C3) | 400bp |
| 9/1 pT181antisense-DT | 547bp |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5m | 30s | 30s | 30s | 30 |
Electrophoresis
Electrophoresis
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | attenuator EcoR1&Spe1 |
| 3 | attenuator negative EcoR1&Spe1 |
| 4 | attenuator Xba1&Pst1 |
| 5 | attenuator negative Xba1&Pst1 |
| 6 | antisense EcoR1&Spe1 |
| 7 | antisense negative EcoR1&Spe1 |
| 8 | 100bp ladder |
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | antisense Xba1&Pst1 |
| 3 | antisense negative Xba1&Pst1 |
| 4 | apt EcoR1&Spe1 |
| 5 | apt negative EcoR1&Spe1 |
| 6 | DT EcoR1&Xba1 |
| 7 | DT negative EcoR1&Xba1 |
| 8 | 100bp ladder |
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | RBS-lysis1 EcoR1&Spe1 |
| 3 | RBS-lysis1 negative |
| 4 | RBS-lysis2 EcoR1&Spe1 |
| 5 | RBS-lysis2 negative |
| 6 | RBS-lysis3 EcoR1&Spe1 |
| 7 | RBS-lysis3 negative |
| 8 | 100bp ladder |
Gel Extraction
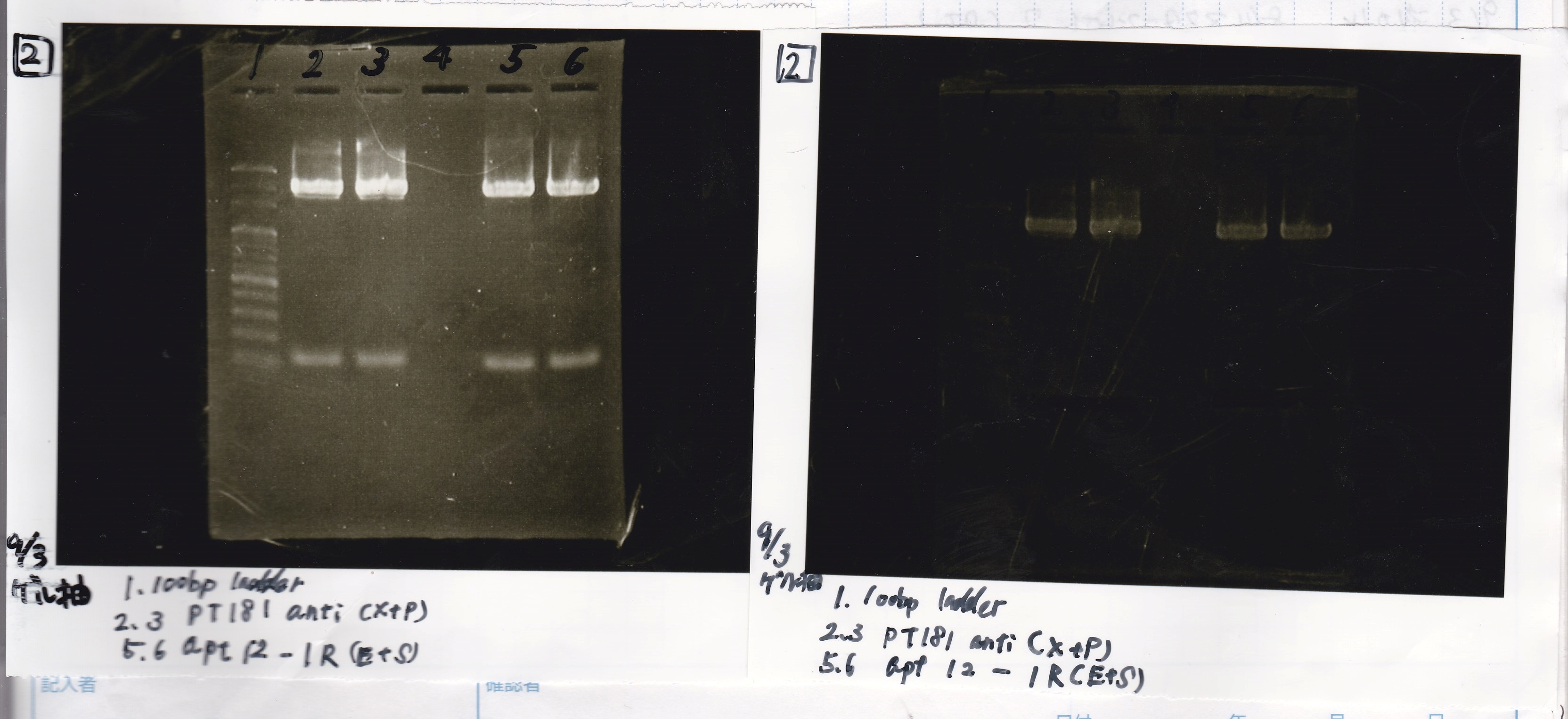
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | |
| 3 | pT181 attenuator | EcoRI&SpeI |
| 4 | pT181 attenuator | EcoRI&SpeI |
| 6 | pT181 attenuator | XbaI&PstI |
| 7 | pT181 attenuator | XbaI&PstI |
| 9 | pT181 antisense | EcoRI&SpeI |
| 10 | pT181 antisense | EcoRI&SpeI |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | |
| 2 | pT181 antisense | XbaI&PstI |
| 3 | pT181 antisense | XbaI&PstI |
| 5 | apt12_1R | EcoRI&SpeI |
| 6 | apt12_1R | EcoRI&SpeI |
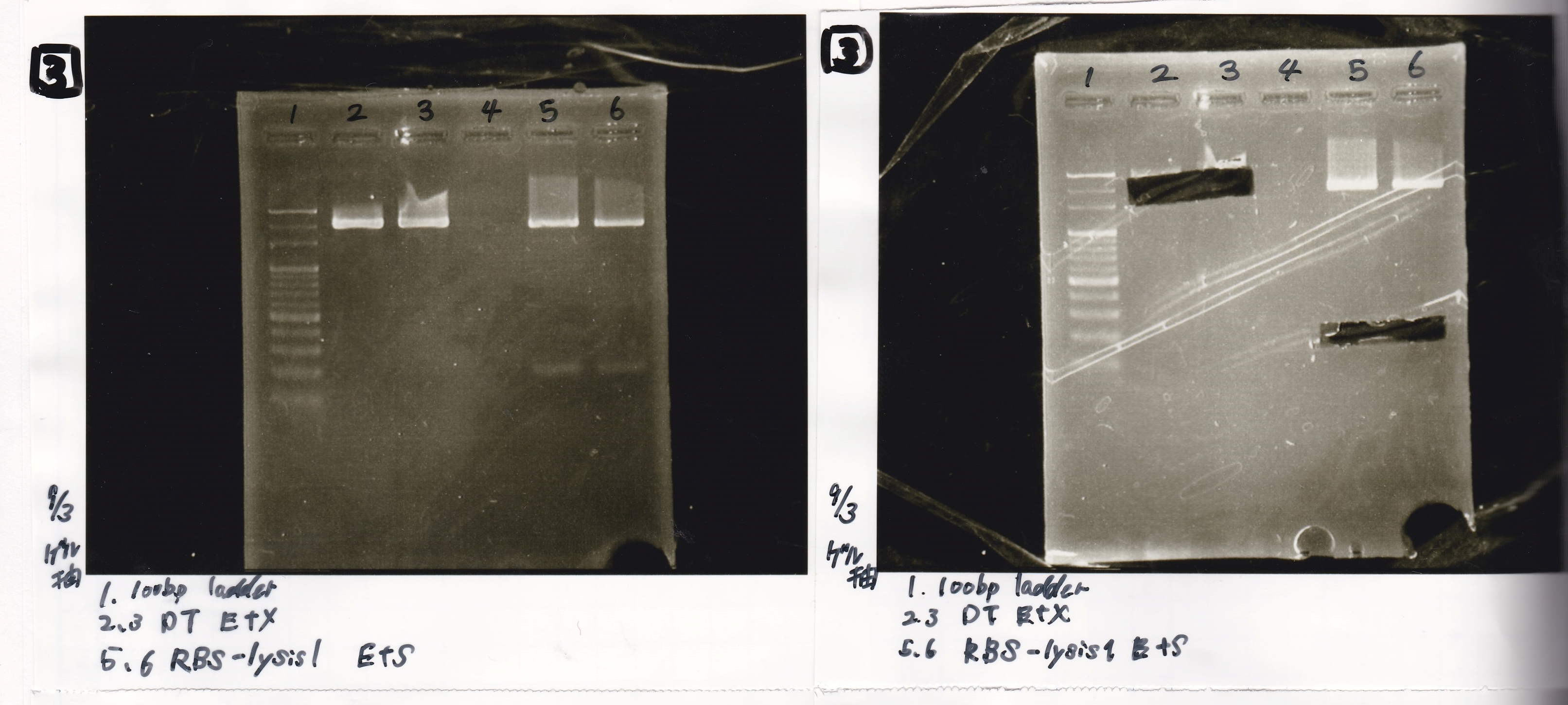
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | |
| 2 | DT | EcoRI&XbaI |
| 3 | DT | EcoRI&XbaI |
| 5 | EcoRI&SpeI | |
| 6 | RBS-lysis1 | EcoRI&SpeI |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | |
| 2 | RBS-lysis2 | EcoRI&SpeI |
| 3 | RBS-lysis2 | EcoRI&SpeI |
| 5 | RBS-lysis3 | EcoRI&SpeI |
| 6 | RBS-lysis3 | EcoRI&SpeI |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| pT181 attenuator(EcoRI&SpeI) | 10.0 | 1.86 | 0.39 |
| pT181 attenuator(XbaI&PstI) | 11.7 | 1.65 | 0.36 |
| pT181 antisense(EcoRI&SpeI) | 4.2 | 1.95 | 0.25 |
| pT181 antisense(XbaI&PstI) | 6.6 | 1.78 | 0.33 |
| tRNA 12_1R apt(EcoRI&SpeI) | 5.7 | 1.73 | 0.29 |
| DT(EcoRI&XbaI) | 16.6 | 1.99 | 0.76 |
| RBS-lysis1(EcoRI&SpeI) | 8.8 | 1.65 | 0.32 |
| RBS-lysis2(EcoRI&SpeI) | 13.4 | 1.61 | 0.48 |
| RBS-lysis3(EcoRI&SpeI) | 10.2 | 2.19 | 0.51 |
Liquid Culture
| Sample | medium |
|---|---|
| 8/16master plate7(DT) | |
| 8/21RBS-lysis1 |
 "
"