Template:Kyoto/Notebook/Sep 2
From 2013.igem.org
(Difference between revisions)
(Created page with "===Liquid Culture=== <div class="experiment"> <span class="author">No name</span>") |
(→Restriction Enzyme Digestion) |
||
| (47 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | ==Sep 2== | ||
| + | |||
| + | ===Colony PCR=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Tatsui</span> | ||
| + | {| class="wikitable" | ||
| + | !|Sample||base pair | ||
| + | |- | ||
| + | |9/1 RBS-lysis2-DT-(1)||985 | ||
| + | |- | ||
| + | |Ptet-RBS-lacZα-DT-(1)||765 | ||
| + | |- | ||
| + | |Plac-RBS-lacZα-DT-(1)||765 | ||
| + | |- | ||
| + | |Plac-RBS-lacZα-DT-(2)||765 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94°C||94°C||55°C||68°C||-- | ||
| + | |- | ||
| + | |5min||30s||30s||1min||30cycles | ||
| + | |} | ||
| + | </div> | ||
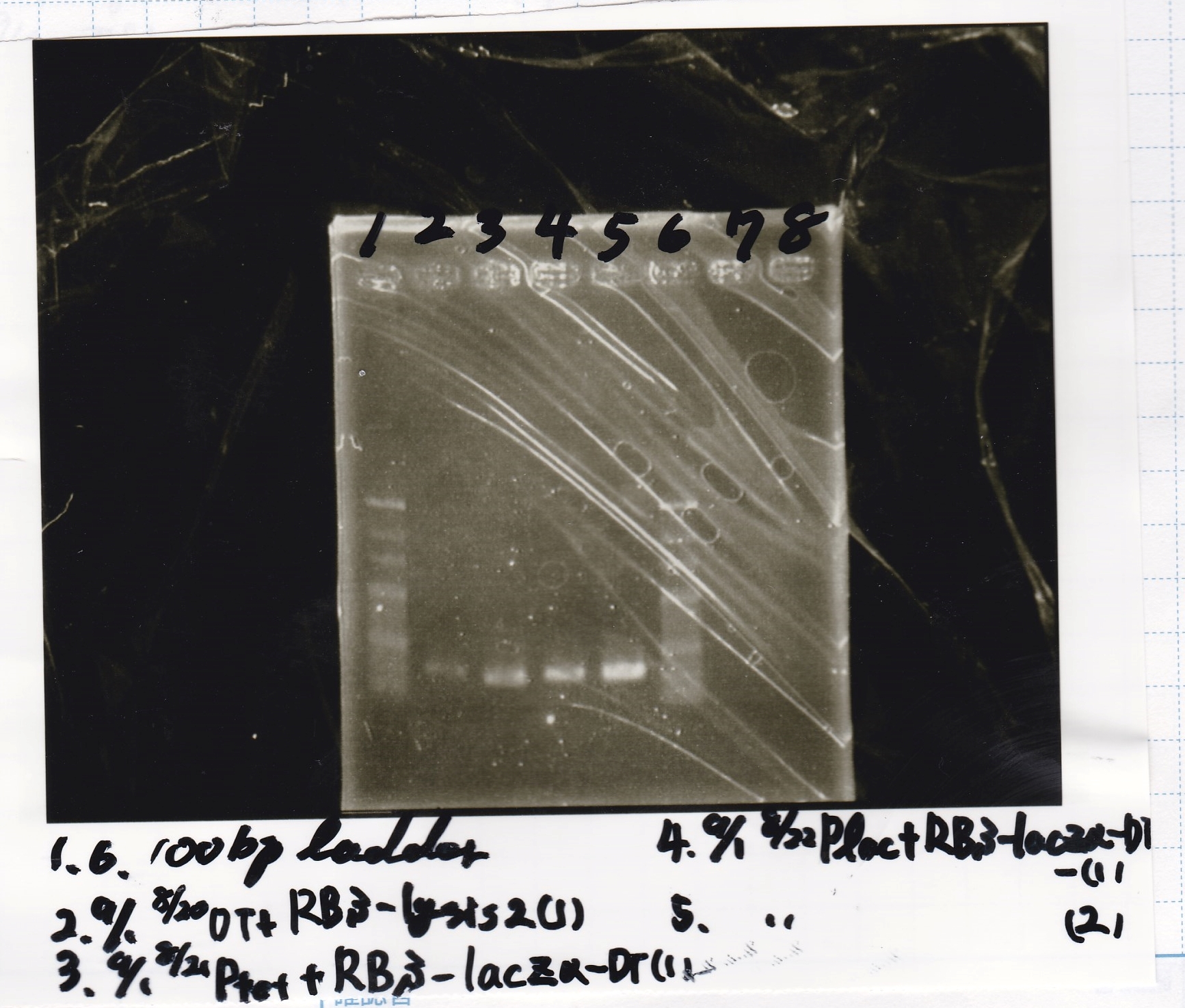
| + | ===Electrophoresis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||100bp ladder | ||
| + | |- | ||
| + | |2||9/1 RBS-lysis2+DT -(1) | ||
| + | |- | ||
| + | |3||9/1 Ptet+RBS-lacZα-DT -(1) | ||
| + | |- | ||
| + | |4||9/1 Plac+RBS-lacZα-DT -(1) | ||
| + | |- | ||
| + | |5||9/1 Plac+RBS-lacZα-DT -(2) | ||
| + | |- | ||
| + | |6||100bp ladder | ||
| + | |} | ||
| + | [[File:Igku Sep2 ColonyPCR (N1-2).jpg]]<br> | ||
| + | </div> | ||
| + | |||
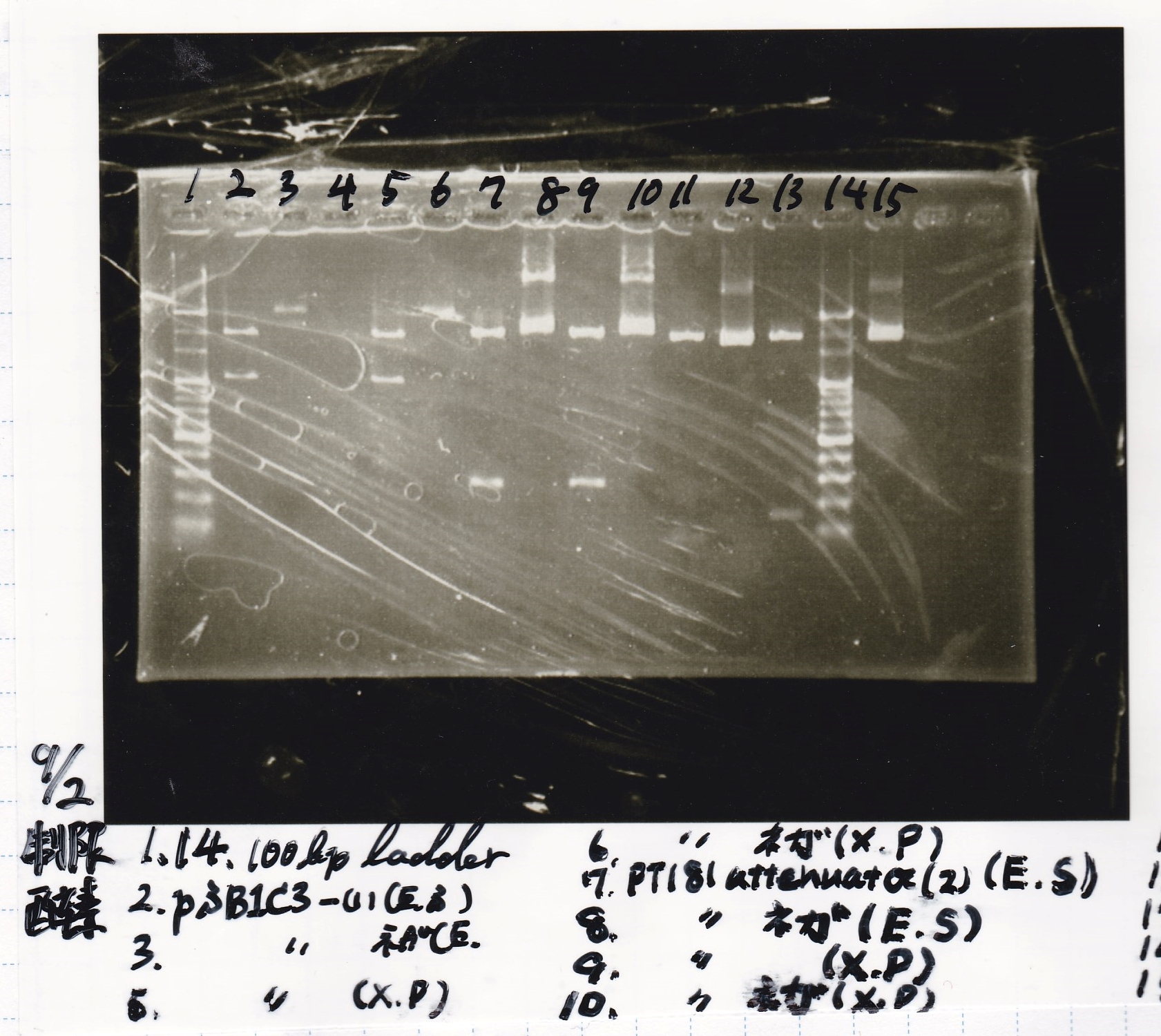
| + | ===Restriction Enzyme Digestion=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto</span> | ||
| + | {| class="wikitable" | ||
| + | ! ||pSB1C3-(1)||EcoRI||SpeI||XbaI||PstI||BufferB||BufferD||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts(EcoRI+SpeI)||7µL||1µL||1µL||0µL||0µL||3µL||0µL||0.3µL||17.7µL||30µL | ||
| + | |- | ||
| + | |NC(EcoRI+SpeI)||0.3µL||0µL||0µL||0µL||0µL||1µL||0µL||0.1µL||8.6µL||10µL | ||
| + | |- | ||
| + | |2 cuts(XbaI+PstI)||7µL||0µL||0µL||1µL||1µL||0µL||3µL||0.3µL||17.7µL||30µL | ||
| + | |- | ||
| + | |NC(XbaI+PstI)||0.3µL||0µL||0µL||0µL||0µL||0µL||1µL||0.1µL||8.6µL||10µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||8/21 tRMA-spinach(1)||EcoRI||SpeI||BufferB||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||8µL||1.0µL||1.0µL||3µL||0.3µL||17.6µL||30µL | ||
| + | |- | ||
| + | |NC||0.5µL||0µL||0µL||1µL||0.1µL||8.4µL||10µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||8/21 tetR aptamer12_1R-(1)||EcoRI||SpeI||BufferB||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||6µL||1.0µL||1.0µL||3µL||0.3µL||18.7µL||30µL | ||
| + | |- | ||
| + | |NC||0.4µL||0µL||0µL||1µL||0.1µL||8.5µL||10µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||8/21 pT181 attenuator-(2)||EcoRI||SpeI||XbaI||PstI||BufferB||BufferD||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts(EcoRI+SpeI)||8µL||1µL||1µL||0µL||0µL||3µL||0µL||0.3µL||16.3µL||30µL | ||
| + | |- | ||
| + | |NC(EcoRI+SpeI)||0.5µL||0µL||0µL||0µL||0µL||1µL||0µL||0.1µL||8.4µL||10µL | ||
| + | |- | ||
| + | |2 cuts(XbaI+PstI)||8µL||0µL||0µL||1µL||1µL||0µL||3µL||0.3µL||16.3µL||30µL | ||
| + | |- | ||
| + | |NC(XbaI+PstI)||0.5µL||0µL||0µL||0µL||0µL||0µL||1µL||0.1µL||8.4µL||10µL | ||
| + | |} | ||
| + | [[File:Igku Sep2 Restriction Enzyme Digestion (N1-1).jpg]]<br> | ||
| + | </div> | ||
| + | |||
===Liquid Culture=== | ===Liquid Culture=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Kojima</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||medium | ||
| + | |- | ||
| + | |tRNA-Spinach-1||Plusgrow medium(+CP) | ||
| + | |- | ||
| + | |tetR aptamaer12_1R-1||Plusgrow medium(+CP) | ||
| + | |- | ||
| + | |pT181 attenuator-1||Plusgrow medium(+CP) | ||
| + | |- | ||
| + | |pT181 antisense-1||Plusgrow medium(+CP) | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Master Plate=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Kojima</span> | ||
| + | {| class="wikitable" | ||
| + | !Number||Use LB plate(+CP) | ||
| + | |- | ||
| + | |1||tRNA Spinach-1 | ||
| + | |- | ||
| + | |2||tetR aptamaer12_1R | ||
| + | |- | ||
| + | |3||tetR aptamaer12_P | ||
| + | |- | ||
| + | |4||tetR aptamaer12_1M | ||
| + | |- | ||
| + | |5||pT181 attenuator-2 | ||
| + | |- | ||
| + | |6||Fusion1 attenuator-1 | ||
| + | |- | ||
| + | |7||Fusion3m2 attenuator-1 | ||
| + | |- | ||
| + | |8||pT181 antisense-1 | ||
| + | |- | ||
| + | |9||Fusion1 antisense-1 | ||
| + | |- | ||
| + | |10||Fuaion6 antisense-1 | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Electrophoresis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto & Tatsui</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||100bp ladder||--||-- | ||
| + | |- | ||
| + | |2||pSB1C3 -(1)||EcoRI||SpeI | ||
| + | |- | ||
| + | |3||pSB1C3 -(1)||--||-- | ||
| + | |- | ||
| + | |5||pSB1C3 -(1)||XbaI||PstI | ||
| + | |- | ||
| + | |6||pSB1C3 -(1)||--||-- | ||
| + | |- | ||
| + | |7||pT181 attenuator (2)||EcoRI||SpeI | ||
| + | |- | ||
| + | |8||pT181 attenuator (2)||--||-- | ||
| + | |- | ||
| + | |9||pT181 attenuator (2)||XbaI||SpeI | ||
| + | |- | ||
| + | |10||pT181 attenuator (2)||--||-- | ||
| + | |- | ||
| + | |11||tetR-aptamer 12_1R||EcoRI||SpeI | ||
| + | |- | ||
| + | |12||tetR-aptamer 12_1R||--||-- | ||
| + | |- | ||
| + | |13||tRNA Spinach (1)||EcoRI||SpeI | ||
| + | |- | ||
| + | |14||100bp ladder||--||-- | ||
| + | |- | ||
| + | |15||tRNA Spinach (1)||--||-- | ||
| + | |} | ||
| + | [[File:Igku Sep2 Restriction Enzyme Digestion (N1-1).jpg]]<br> | ||
| + | </div> | ||
| + | |||
| + | ===Gel Extraction=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||100bp ladder||- | ||
| + | |- | ||
| + | |2||rowspan="2"|pSB1C3(EcoRI+SpeI)||rowspan="2"|EcoRI&SpeI | ||
| + | |- | ||
| + | |3 | ||
| + | |- | ||
| + | |4||--||-- | ||
| + | |- | ||
| + | |5||rowspan="2"|8/24 tetR aptamer 12_1R||rowspan="2"|EcoRI&SpeI | ||
| + | |- | ||
| + | |6 | ||
| + | |} | ||
| + | [[File:Igku Sep1 Gel Extraction(N2-1).jpg]]<br> | ||
| + | [[File:Igku Sep1 Gel Extraction(N2-2).jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||100bp ladder||- | ||
| + | |- | ||
| + | |2||rowspan="2"|pT181 attenuator(2)(EcoRI+SpeI)||rowspan="2"|EcoRI&SpeI | ||
| + | |- | ||
| + | |3 | ||
| + | |- | ||
| + | |4||--||-- | ||
| + | |- | ||
| + | |5||rowspan="2"|pT181 attenuator(2)(XbaI+PstI)||rowspan="2"|EcoRI&SpeI | ||
| + | |- | ||
| + | |6 | ||
| + | |- | ||
| + | |7||--||-- | ||
| + | |- | ||
| + | |8||rowspan="2"|pT181 attenuator(2)(XbaI+PstI)||rowspan="2"|EcoRI&SpeI | ||
| + | |- | ||
| + | |9 | ||
| + | |- | ||
| + | |10||--||-- | ||
| + | |- | ||
| + | |11||rowspan="2"|Spinach||rowspan="2"|EcoRI&SpaI | ||
| + | |- | ||
| + | |12 | ||
| + | |} | ||
| + | [[File:Igku Sep1 Gel Extraction(N3-1).jpg]]<br> | ||
| + | [[File:Igku Sep1 Gel Extraction(N3-2).jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Name||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |pSB1C3 (EcoRI&SpeI)||3.0||2.18||0.31 | ||
| + | |- | ||
| + | |pSB1C3 (XbaI&PstI)||4.7||2.25||0.36 | ||
| + | |- | ||
| + | |pT181 attenuator-(2)(EcoR&SpeI)||8.6||2.74||0.01 | ||
| + | |- | ||
| + | |pT181 attenuator-(2) (XbaI&PstI)||16.5||2.46||0.03 | ||
| + | |- | ||
| + | |Spinach (EcoRI&SpeI)||2.8||2.98||0.27 | ||
| + | |- | ||
| + | |tetR aptamer12_1R (EcoRI&SpeI)||50.4||28.07||1.97 | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Colony PCR=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !|Sample||base pair | ||
| + | |- | ||
| + | |9/1 Spinach(pSB1C3)||about 450 | ||
| + | |- | ||
| + | |9/1 Spinach-DT||596 | ||
| + | |- | ||
| + | |9/1 RBS-lysis1-DT||613 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94°C||94°C||55°C||68°C||-- | ||
| + | |- | ||
| + | |5min||30s||30s||40s||30cycles | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !|Sample||base pair | ||
| + | |- | ||
| + | |9/1 Ptet-RBS-lacZα-DT-2||738 | ||
| + | |- | ||
| + | |9/1 Ptet-RBS-lacZα-DT-3||738 | ||
| + | |- | ||
| + | |9/1 Ptet-RBS-lacZα-DT-4||738 | ||
| + | |- | ||
| + | |9/1 Ptet-RBS-lacZα-DT-5||738 | ||
| + | |- | ||
| + | |9/1 Ptet-RBS-lacZα-DT-6||738 | ||
| + | |- | ||
| + | |9/1 RBS-lysis2-DT-2||985 | ||
| + | |- | ||
| + | |9/1 RBS-lysis2-DT-3||985 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94°C||94°C||55°C||68°C||-- | ||
| + | |- | ||
| + | |5min||30s||30s||1min||30cycles | ||
| + | |} | ||
| + | [[File:Igku Sep3 ColonyPCR (N1).jpg]] | ||
| + | </div> | ||
| + | |||
| + | ===Electrophoresis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||100bp ladder | ||
| + | |- | ||
| + | |2||Spinach(pSB1C3) | ||
| + | |- | ||
| + | |3||Spinach-DT | ||
| + | |- | ||
| + | |4||RBS-lysis1-DT | ||
| + | |- | ||
| + | |5||100bp ladder | ||
| + | |} | ||
| + | [[File:Igku Sep2 ColonyPCR (N4).jpg]]<br> | ||
| + | </div> | ||
| + | |||
| + | ===Master Plate=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Number||Use LB plate(+CP) | ||
| + | |- | ||
| + | |1||9/1 Spinach(pSB1C3) | ||
| + | |- | ||
| + | |2||9/1 Spinach-DT-(1) | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Liquid Culture=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||medium | ||
| + | |- | ||
| + | |pT181 attenuator||Plusgrow medium (+CP) | ||
| + | |- | ||
| + | |pT181 antisense||Plusgrow medium (+CP) | ||
| + | |- | ||
| + | |Spinach||Plusgrow medium (+CP) | ||
| + | |} | ||
| + | *incubated at 37 °C for 1 hour | ||
| + | </div> | ||
| + | |||
| + | ===Transformation=== | ||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">No name</span> | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Name||Sample||Competent Cells||Total||Plate | ||
| + | |- | ||
| + | |8/28 RBS-lysis3-DT||3µL||30µL||33µL||CP | ||
| + | |} | ||
| + | </div> | ||
Latest revision as of 18:50, 25 September 2013
Contents |
Sep 2
Colony PCR
| Sample | base pair |
|---|---|
| 9/1 RBS-lysis2-DT-(1) | 985 |
| Ptet-RBS-lacZα-DT-(1) | 765 |
| Plac-RBS-lacZα-DT-(1) | 765 |
| Plac-RBS-lacZα-DT-(2) | 765 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 1min | 30cycles |
Electrophoresis
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | 9/1 RBS-lysis2+DT -(1) |
| 3 | 9/1 Ptet+RBS-lacZα-DT -(1) |
| 4 | 9/1 Plac+RBS-lacZα-DT -(1) |
| 5 | 9/1 Plac+RBS-lacZα-DT -(2) |
| 6 | 100bp ladder |
Restriction Enzyme Digestion
| pSB1C3-(1) | EcoRI | SpeI | XbaI | PstI | BufferB | BufferD | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 cuts(EcoRI+SpeI) | 7µL | 1µL | 1µL | 0µL | 0µL | 3µL | 0µL | 0.3µL | 17.7µL | 30µL |
| NC(EcoRI+SpeI) | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 0µL | 0.1µL | 8.6µL | 10µL |
| 2 cuts(XbaI+PstI) | 7µL | 0µL | 0µL | 1µL | 1µL | 0µL | 3µL | 0.3µL | 17.7µL | 30µL |
| NC(XbaI+PstI) | 0.3µL | 0µL | 0µL | 0µL | 0µL | 0µL | 1µL | 0.1µL | 8.6µL | 10µL |
| 8/21 tRMA-spinach(1) | EcoRI | SpeI | BufferB | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 8µL | 1.0µL | 1.0µL | 3µL | 0.3µL | 17.6µL | 30µL |
| NC | 0.5µL | 0µL | 0µL | 1µL | 0.1µL | 8.4µL | 10µL |
| 8/21 tetR aptamer12_1R-(1) | EcoRI | SpeI | BufferB | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 6µL | 1.0µL | 1.0µL | 3µL | 0.3µL | 18.7µL | 30µL |
| NC | 0.4µL | 0µL | 0µL | 1µL | 0.1µL | 8.5µL | 10µL |
| 8/21 pT181 attenuator-(2) | EcoRI | SpeI | XbaI | PstI | BufferB | BufferD | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 cuts(EcoRI+SpeI) | 8µL | 1µL | 1µL | 0µL | 0µL | 3µL | 0µL | 0.3µL | 16.3µL | 30µL |
| NC(EcoRI+SpeI) | 0.5µL | 0µL | 0µL | 0µL | 0µL | 1µL | 0µL | 0.1µL | 8.4µL | 10µL |
| 2 cuts(XbaI+PstI) | 8µL | 0µL | 0µL | 1µL | 1µL | 0µL | 3µL | 0.3µL | 16.3µL | 30µL |
| NC(XbaI+PstI) | 0.5µL | 0µL | 0µL | 0µL | 0µL | 0µL | 1µL | 0.1µL | 8.4µL | 10µL |
Liquid Culture
| Sample | medium |
|---|---|
| tRNA-Spinach-1 | Plusgrow medium(+CP) |
| tetR aptamaer12_1R-1 | Plusgrow medium(+CP) |
| pT181 attenuator-1 | Plusgrow medium(+CP) |
| pT181 antisense-1 | Plusgrow medium(+CP) |
Master Plate
| Number | Use LB plate(+CP) |
|---|---|
| 1 | tRNA Spinach-1 |
| 2 | tetR aptamaer12_1R |
| 3 | tetR aptamaer12_P |
| 4 | tetR aptamaer12_1M |
| 5 | pT181 attenuator-2 |
| 6 | Fusion1 attenuator-1 |
| 7 | Fusion3m2 attenuator-1 |
| 8 | pT181 antisense-1 |
| 9 | Fusion1 antisense-1 |
| 10 | Fuaion6 antisense-1 |
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | pSB1C3 -(1) | EcoRI | SpeI |
| 3 | pSB1C3 -(1) | -- | -- |
| 5 | pSB1C3 -(1) | XbaI | PstI |
| 6 | pSB1C3 -(1) | -- | -- |
| 7 | pT181 attenuator (2) | EcoRI | SpeI |
| 8 | pT181 attenuator (2) | -- | -- |
| 9 | pT181 attenuator (2) | XbaI | SpeI |
| 10 | pT181 attenuator (2) | -- | -- |
| 11 | tetR-aptamer 12_1R | EcoRI | SpeI |
| 12 | tetR-aptamer 12_1R | -- | -- |
| 13 | tRNA Spinach (1) | EcoRI | SpeI |
| 14 | 100bp ladder | -- | -- |
| 15 | tRNA Spinach (1) | -- | -- |
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | - |
| 2 | pSB1C3(EcoRI+SpeI) | EcoRI&SpeI |
| 3 | ||
| 4 | -- | -- |
| 5 | 8/24 tetR aptamer 12_1R | EcoRI&SpeI |
| 6 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | - |
| 2 | pT181 attenuator(2)(EcoRI+SpeI) | EcoRI&SpeI |
| 3 | ||
| 4 | -- | -- |
| 5 | pT181 attenuator(2)(XbaI+PstI) | EcoRI&SpeI |
| 6 | ||
| 7 | -- | -- |
| 8 | pT181 attenuator(2)(XbaI+PstI) | EcoRI&SpeI |
| 9 | ||
| 10 | -- | -- |
| 11 | Spinach | EcoRI&SpaI |
| 12 |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| pSB1C3 (EcoRI&SpeI) | 3.0 | 2.18 | 0.31 |
| pSB1C3 (XbaI&PstI) | 4.7 | 2.25 | 0.36 |
| pT181 attenuator-(2)(EcoR&SpeI) | 8.6 | 2.74 | 0.01 |
| pT181 attenuator-(2) (XbaI&PstI) | 16.5 | 2.46 | 0.03 |
| Spinach (EcoRI&SpeI) | 2.8 | 2.98 | 0.27 |
| tetR aptamer12_1R (EcoRI&SpeI) | 50.4 | 28.07 | 1.97 |
Colony PCR
| Sample | base pair |
|---|---|
| 9/1 Spinach(pSB1C3) | about 450 |
| 9/1 Spinach-DT | 596 |
| 9/1 RBS-lysis1-DT | 613 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 40s | 30cycles |
| Sample | base pair |
|---|---|
| 9/1 Ptet-RBS-lacZα-DT-2 | 738 |
| 9/1 Ptet-RBS-lacZα-DT-3 | 738 |
| 9/1 Ptet-RBS-lacZα-DT-4 | 738 |
| 9/1 Ptet-RBS-lacZα-DT-5 | 738 |
| 9/1 Ptet-RBS-lacZα-DT-6 | 738 |
| 9/1 RBS-lysis2-DT-2 | 985 |
| 9/1 RBS-lysis2-DT-3 | 985 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 1min | 30cycles |
Electrophoresis
Master Plate
| Number | Use LB plate(+CP) |
|---|---|
| 1 | 9/1 Spinach(pSB1C3) |
| 2 | 9/1 Spinach-DT-(1) |
Liquid Culture
| Sample | medium |
|---|---|
| pT181 attenuator | Plusgrow medium (+CP) |
| pT181 antisense | Plusgrow medium (+CP) |
| Spinach | Plusgrow medium (+CP) |
- incubated at 37 °C for 1 hour
Transformation
| Name | Sample | Competent Cells | Total | Plate |
|---|---|---|---|---|
| 8/28 RBS-lysis3-DT | 3µL | 30µL | 33µL | CP |
 "
"