Team:Colombia Uniandes/ChimiJournal
From 2013.igem.org
Trafalmejo (Talk | contribs) |
(→September 4th) |
||
| (26 intermediate revisions not shown) | |||
| Line 28: | Line 28: | ||
==== '''15th June 2013''' ==== | ==== '''15th June 2013''' ==== | ||
| - | We | + | We picked the transformant colonies. |
| - | + | ||
===='''18th June 2013'''==== | ===='''18th June 2013'''==== | ||
| Line 65: | Line 64: | ||
<li>Resuspend in 40 µL miliQ water.</li> | <li>Resuspend in 40 µL miliQ water.</li> | ||
</ol> | </ol> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
===='''June 26, 2013'''==== | ===='''June 26, 2013'''==== | ||
| Line 99: | Line 86: | ||
<p align="justify"> | <p align="justify"> | ||
We repeated the PCR for A and used lambda phage DNA for carrier DNA. | We repeated the PCR for A and used lambda phage DNA for carrier DNA. | ||
| + | We also tried extracting yeast genome using a modified Harju “Bust n’ Grab” protocol using three parallel methods: | ||
| + | <ul> | ||
| + | <li>Method “H” used the regular lysis buffer.</li> | ||
| + | <li>Method “C” used the following lysis buffer: 3% Triton, 100 mM LiCl, 10 mM Tris-HCl, 1 mM EDTA, 100 mM NaAc.</li> | ||
| + | <li>Method “O” used the following lysis buffer: 2% triton, 1& SDS, 10 mM tris-HCl, 1 mM EDTA, 100 mM LiCl.</li> | ||
| + | </ul> | ||
| + | </p> | ||
<p align="justify"> | <p align="justify"> | ||
Conformation gel was run with wells: | Conformation gel was run with wells: | ||
| Line 109: | Line 103: | ||
<li>Method O</li> | <li>Method O</li> | ||
</ol> | </ol> | ||
| + | The genome extraction still isn't working! :( | ||
</p> | </p> | ||
| Line 131: | Line 126: | ||
<li>Hold (4 °C, indefinite time)</li> | <li>Hold (4 °C, indefinite time)</li> | ||
</ol></li> | </ol></li> | ||
| - | |||
| - | |||
| - | |||
| - | |||
===='''July 2nd, 2013'''==== | ===='''July 2nd, 2013'''==== | ||
| - | + | Still trying to successfully extract the yeast genome! This time we tried an alternate method where we used two different solutions to break the cell wall: | |
<ul> | <ul> | ||
| - | <li>Solution I: Glucose 50 mM, EDTA pH 8 10 mM, Tris-HCl pH 8 25 mM (esterilized)</li> | + | <li>Solution I: Glucose 50 mM, EDTA pH 8 10 mM, Tris-HCl pH 8 25 mM (esterilized) + Zymolyase</li> |
<li>Solution II: NaOH 0.2 N, SPS 1%</li> | <li>Solution II: NaOH 0.2 N, SPS 1%</li> | ||
| + | The rest of the protocol was taken from GenElute DNA Kit from Sigma-Aldrich. | ||
</ul> | </ul> | ||
| + | |||
| + | ===='''July 3rd, 2013'''==== | ||
| + | The genome extraction was better, but it's mostly degraded DNA! We still have to improve the protocol. | ||
===='''July 5th, 2013'''==== | ===='''July 5th, 2013'''==== | ||
| - | We | + | We're still improving our genome extraction protocol. This time we're trying 4 variations to break the cell wall |
| - | <br> | + | <br>We're varying the incubation of both solutions, the one that comes with the kit (Proteinase K + Lysis buffer) and the zymolyase solution we previously used. The four variations were as follows. |
<ul> | <ul> | ||
<li>A: Zymolyase for ½ h at 37 °C</li> | <li>A: Zymolyase for ½ h at 37 °C</li> | ||
<li>B: Zymolyase for ½ h at 37 °C, then protease K + lysis T ½ h at 55 °C</li> | <li>B: Zymolyase for ½ h at 37 °C, then protease K + lysis T ½ h at 55 °C</li> | ||
<li>C: Zymolyase for ½ h at 37 °C, then protease K + lysis T ½ h at 37 °C</li> | <li>C: Zymolyase for ½ h at 37 °C, then protease K + lysis T ½ h at 37 °C</li> | ||
| - | <li>D: | + | <li>D: Regular GenElute Genome extraction protocol</li> |
</ul> | </ul> | ||
| + | The rest of the steps were done following the instructions from the GenElute Genome extraction protocol. | ||
| - | <br>We ran a gel in this order: WM, A, B, C, D. | + | <br>We ran a gel in this order: WM, A, B, C, D. |
| + | <br>Both B and C gave results, with C giving better yields! We're keeping the C protocol and we're happy we can start extracting parts from the yeast genome! | ||
| + | <br> | ||
<br>We performed PCRs for BAP2, GAL4, yeast terminator and pGAL1. These were the conditions: | <br>We performed PCRs for BAP2, GAL4, yeast terminator and pGAL1. These were the conditions: | ||
<br>PCR fusion 2 steps*, with process 3 at 72 °C for 45 s | <br>PCR fusion 2 steps*, with process 3 at 72 °C for 45 s | ||
| Line 169: | Line 167: | ||
Today we ran PCRs for pGAL1 and mCherry. Now that we have several parts, we must have a clear understanding of our notation! | Today we ran PCRs for pGAL1 and mCherry. Now that we have several parts, we must have a clear understanding of our notation! | ||
<ul> | <ul> | ||
| - | < | + | <li>pBap2 = C</li> |
<li>GAL4 = D</li> | <li>GAL4 = D</li> | ||
<li>VP16 = A</li> | <li>VP16 = A</li> | ||
| Line 217: | Line 215: | ||
In our quest to obtain again all our parts, we performed the following fusion PCRs: ABF1 (primers 1 & 31), GF2 (primers 17 & 31), E1GF2 (primers 15 & 31), E2G (primers 15 & 13), E3G (primers 15 & 13), E4G (primers 15 & 13). | In our quest to obtain again all our parts, we performed the following fusion PCRs: ABF1 (primers 1 & 31), GF2 (primers 17 & 31), E1GF2 (primers 15 & 31), E2G (primers 15 & 13), E3G (primers 15 & 13), E4G (primers 15 & 13). | ||
| - | We also performed the | + | We also performed the transformation by TOPO cloning. |
| + | |||
| + | ===='''September 4th, 2013'''==== | ||
| + | <p align="justify"> | ||
| + | We inoculated cells with dexamethasone. The original syringe had an initial concentration of 8mg/2mL. We obtained an initial volume of 3.189*10^-2 µL, so we had to dilute first the dexamethasone: 9900 µL water + 100 µL dexametasona.</p> | ||
| + | |||
| + | <p align="justify">Induction experiment ON: 5 mL LB + 20 µL ampicillin + 20 µL kanamycin + 10 µL inoculum + 32 µL dexamethasone (1:1000) (inoculate ON).</p> | ||
| + | |||
| + | <p align="justify"> | ||
| + | Gel PCR: F-H-P-E1GF2-MP-E2GF-E3GF1-E3GF2-E4GF- Everything was succesful except E1GF2.</p> | ||
| + | <p align="justify"> | ||
| + | We digested the obtained PCRs with 5 µL Buffer CutSmart + 15 µL PCR +1 µL XbaI + 1 µL SpeI + 28 µL water (for each PCR).</p> | ||
| + | |||
<html> | <html> | ||
</div> | </div> | ||
</div> | </div> | ||
</html> | </html> | ||
Latest revision as of 06:05, 27 September 2013
Hands at work!
Here you will find the overall progression of our work at the laboratory designing Chimi.
13th June 2013
The first thing we did was to extract the plasmids from the iGEM plaque.
- From the 2013 kit, Plate 1, Well 19 – o
- From the 2013 kit, Plate 1, Well 2 – i
- From the 2013 kit, Plate 3, Well 17 – c
The iGEM parts were taken in order to perform an electroporation. For this, we use:
- 20 µ of miliQ water (ultra pure). Find the plate and stick the tip with water into the well, perforating the aluminum.
- Resuspend the well’s content by gentle pipetting.
- When the water has a dark red color, transfer it to a PCR eppendorf and put on ice.
15th June 2013
We picked the transformant colonies.
18th June 2013
We performed miniprep procedures with the GenElute HP Plasmid Miniprep kit.
This are the overall steps:
- Harvest cells.
- Resuspend cells.
- Cell lysis.
- Neutralization.
Spin method:
- Prepare column.
- Load cleared lysate.
- Wash column with wash solution 1.
- Was column with wash solution 2.
- Centrifuge.
- Elute DNA.
We did a confirmation Gel 100 V x 30 min. -> It showed 1 bond in the first two wells corresponding to Nal1 and Nal2. We succesfully extracted the Nal plasmids!
June 21st, 2013
Harju et al., “Bust n’ Grab” Protocol for Yeast Genomic DNA Extraction
- 5 mL of overnight culture of S. cerevisiae (in BHI medium) were centrifuged at 8500 rpm for 5 min. Discard de supernatant.
- 500 µL of Harju lysis buffer were added to each tube.
- Place 2 min at -20 °C, 1 min in water bath at 90 °C and repeat.
- Vortex 30 s.
- Add 500 µL of chloroform, vortex 2 min and centrifuge 3 min at 8500 rpm.
- Transfer the upper aqueous phase to a tube with 800 µL of chilled 100% ethanol and mix by inversion.
- Incubate for 5 min at room temperature or at 30 °C.
- Centrifuge for 5 min, 8500 rpm, and discard supernatant.
- Wash the pellet with 500 µL of ethanol (100%) by vortex. Repeat step 9.
- Dry pellets at room temperature or at 60 °C.
- Resuspend in 40 µL miliQ water.
June 26, 2013
- We made competent yeast following the procedure mentioned before.
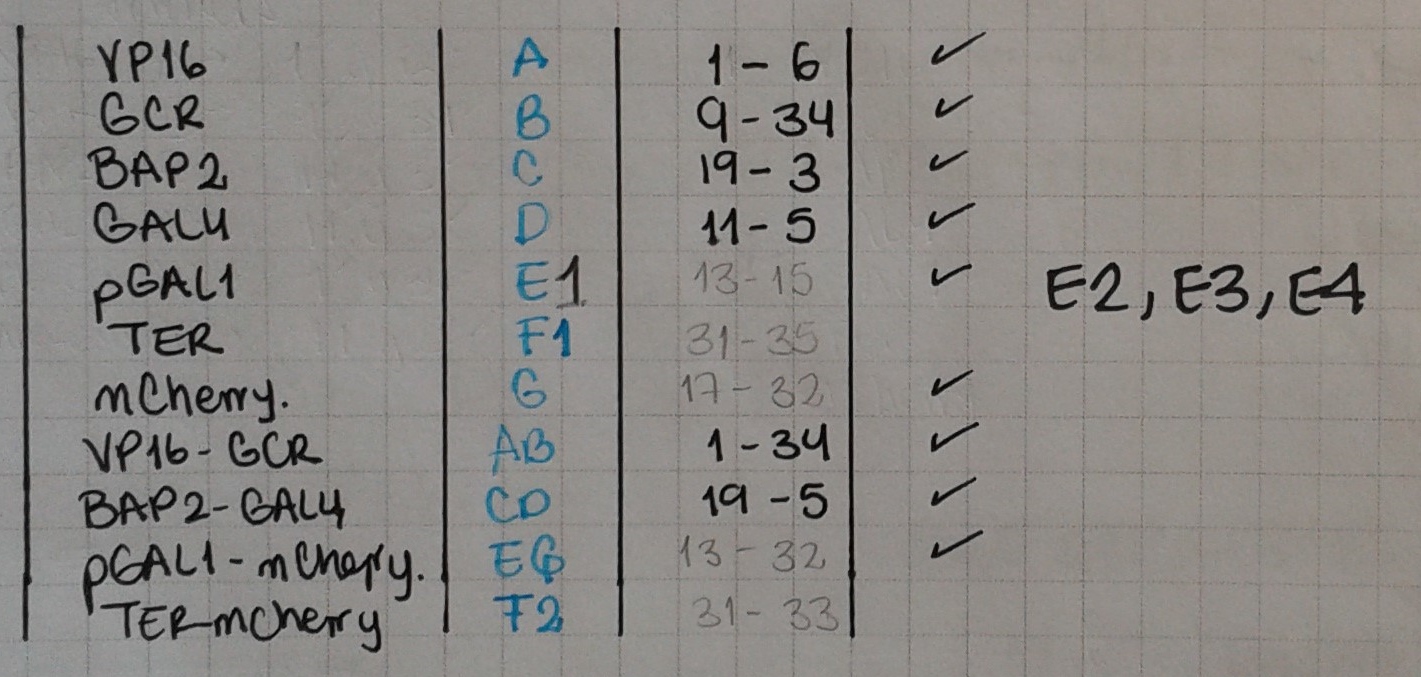
- We also made our first PCRs! We used primers 6 & 1 (A) and 34 & 9 (B) to extract VP16 and GCR from the Nal1 plasmid.
- Confirmation gel (2013-06-26 19 hr 16 min.jpg & 2013-06-26 19hr 15 min.jpg) with wells:
- Ladder
- PCR A
- PCR B
- Miniprep for Nal. 1
A = VP16
B = GCR
June 27, 2013
We repeated the PCR for A and used lambda phage DNA for carrier DNA.
We also tried extracting yeast genome using a modified Harju “Bust n’ Grab” protocol using three parallel methods:
- Method “H” used the regular lysis buffer.
- Method “C” used the following lysis buffer: 3% Triton, 100 mM LiCl, 10 mM Tris-HCl, 1 mM EDTA, 100 mM NaAc.
- Method “O” used the following lysis buffer: 2% triton, 1& SDS, 10 mM tris-HCl, 1 mM EDTA, 100 mM LiCl.
Conformation gel was run with wells:
- Ladder
- PCR A (repeated)
- Carrier lambda PCR
- Method C
- Method H
- Method O
The genome extraction still isn't working! :(
We then ran a fusion PCR with GAL4 and VP16 (A and B). These were the PCR conditions:
- 1st PCR --> 2-step PCR
Cycle steps
- Initial denaturation (98 °C, 30 s)
- 15 cycles (98 °C, 10 s; 72 °C, 35 s)
- Final extension (72 °C, 5 min)
- Hold (4 °C, indefinite time)
- 2nd PCR --> 2-step PCR (add primers)
Cycle steps
- Initial denaturation (98 °C, 30 s)
- 35 cycles (98 °C, 10 s; 72 °C, 35 s)
- Final extension (72 °C, 10 min)
- Hold (4 °C, indefinite time)
July 2nd, 2013
Still trying to successfully extract the yeast genome! This time we tried an alternate method where we used two different solutions to break the cell wall:
- Solution I: Glucose 50 mM, EDTA pH 8 10 mM, Tris-HCl pH 8 25 mM (esterilized) + Zymolyase
- Solution II: NaOH 0.2 N, SPS 1%
The rest of the protocol was taken from GenElute DNA Kit from Sigma-Aldrich.
July 3rd, 2013
The genome extraction was better, but it's mostly degraded DNA! We still have to improve the protocol.
July 5th, 2013
We're still improving our genome extraction protocol. This time we're trying 4 variations to break the cell wall
We're varying the incubation of both solutions, the one that comes with the kit (Proteinase K + Lysis buffer) and the zymolyase solution we previously used. The four variations were as follows.
- A: Zymolyase for ½ h at 37 °C
- B: Zymolyase for ½ h at 37 °C, then protease K + lysis T ½ h at 55 °C
- C: Zymolyase for ½ h at 37 °C, then protease K + lysis T ½ h at 37 °C
- D: Regular GenElute Genome extraction protocol
The rest of the steps were done following the instructions from the GenElute Genome extraction protocol.
We ran a gel in this order: WM, A, B, C, D.
Both B and C gave results, with C giving better yields! We're keeping the C protocol and we're happy we can start extracting parts from the yeast genome!
We performed PCRs for BAP2, GAL4, yeast terminator and pGAL1. These were the conditions:
PCR fusion 2 steps*, with process 3 at 72 °C for 45 s
*
- 98 °C, 1 min
- 98 °C, 10 s
- 72 °C, 45 s
Steps 2 and 3 x 35
- 72 °C, 10 min
- 4 °C
July 9th, 2013
Today we ran PCRs for pGAL1 and mCherry. Now that we have several parts, we must have a clear understanding of our notation!
- pBap2 = C
- GAL4 = D
- VP16 = A
- GCR = B
- TER = F
- VP16 + GCR = AB
- pBAP2 + GAL4 = CD
- AB + TER = ABF
July 18th, 2013
Today we ran PCRs for the following fusions: F1 = TER-GCR, with primers 31 & 35; F2 = TER – mCherry, with primers 31 & 33. We also amplified E = pGAL1, using primers 13 & 15.
July 19th, 2013
Today we amplified F2 that we obtained yesterday.
July 22nd, 2013
Today we tried to fuse C-D using primers 19 and 5, TER-GCR using primers 31 and 35 and TER-mCherry using primers 31 and 33. After 15 PCR cycles, we added the primers.
July 24th, 2013
Today we extracted the terminator from the yeast genome (F1 and F2) and pGAL1 from miniprep (E). We also did PCRs to fuse again our big parts: pBAP2 – GAL4 – VP16 – GCR (CD-AB) (primers 19 & 34) and pGAL1 – mCherry (E-G) (primers 32 & 15).
At the moment, this is our progress!:
August 5th, 2013
Things are advancing at a fast pace! Today we did miniprep to obtain PUC19!
August 6th, 2013
After doing a confirmation gel, C and CD are no longer with us! So we need to repeat them!
August 16th, 2013
Still no C nor D, so we did PCRs again with different methods to obtain C1, C2, D1 and D2.
September 3rd, 2013
Today we confirmed what we will be sending for the regional competition!
qBlocks (200 ng):
- Terminator (114486764) (F1 & F2)
- 3X UAS-pGAL1 (114486765) (E2)
- 6X UAS-pGAL1 (114486766) (E3)
- 9X UAS-pGAL1 (114486767) (E4)
Final volume: (40 µL)
Final concentration: 5 ng/µL
In our quest to obtain again all our parts, we performed the following fusion PCRs: ABF1 (primers 1 & 31), GF2 (primers 17 & 31), E1GF2 (primers 15 & 31), E2G (primers 15 & 13), E3G (primers 15 & 13), E4G (primers 15 & 13).
We also performed the transformation by TOPO cloning.
September 4th, 2013
<p align="justify">
We inoculated cells with dexamethasone. The original syringe had an initial concentration of 8mg/2mL. We obtained an initial volume of 3.189*10^-2 µL, so we had to dilute first the dexamethasone: 9900 µL water + 100 µL dexametasona.</p>
<p align="justify">Induction experiment ON: 5 mL LB + 20 µL ampicillin + 20 µL kanamycin + 10 µL inoculum + 32 µL dexamethasone (1:1000) (inoculate ON).</p>
<p align="justify">
Gel PCR: F-H-P-E1GF2-MP-E2GF-E3GF1-E3GF2-E4GF- Everything was succesful except E1GF2.</p>
<p align="justify">
We digested the obtained PCRs with 5 µL Buffer CutSmart + 15 µL PCR +1 µL XbaI + 1 µL SpeI + 28 µL water (for each PCR).</p>
Hands at work!
Here you will find the overall progression of our work at the laboratory designing Chimi.
13th June 2013
The first thing we did was to extract the plasmids from the iGEM plaque.
- From the 2013 kit, Plate 1, Well 19 – o
- From the 2013 kit, Plate 1, Well 2 – i
- From the 2013 kit, Plate 3, Well 17 – c
The iGEM parts were taken in order to perform an electroporation. For this, we use:
- 20 µ of miliQ water (ultra pure). Find the plate and stick the tip with water into the well, perforating the aluminum.
- Resuspend the well’s content by gentle pipetting.
- When the water has a dark red color, transfer it to a PCR eppendorf and put on ice.
15th June 2013
We picked the transformant colonies.
18th June 2013
We performed miniprep procedures with the GenElute HP Plasmid Miniprep kit.
This are the overall steps:
- Harvest cells.
- Resuspend cells.
- Cell lysis.
- Neutralization.
- Prepare column.
- Load cleared lysate.
- Wash column with wash solution 1.
- Was column with wash solution 2.
- Centrifuge.
- Elute DNA.
Spin method:
We did a confirmation Gel 100 V x 30 min. -> It showed 1 bond in the first two wells corresponding to Nal1 and Nal2. We succesfully extracted the Nal plasmids!
June 21st, 2013
Harju et al., “Bust n’ Grab” Protocol for Yeast Genomic DNA Extraction
- 5 mL of overnight culture of S. cerevisiae (in BHI medium) were centrifuged at 8500 rpm for 5 min. Discard de supernatant.
- 500 µL of Harju lysis buffer were added to each tube.
- Place 2 min at -20 °C, 1 min in water bath at 90 °C and repeat.
- Vortex 30 s.
- Add 500 µL of chloroform, vortex 2 min and centrifuge 3 min at 8500 rpm.
- Transfer the upper aqueous phase to a tube with 800 µL of chilled 100% ethanol and mix by inversion.
- Incubate for 5 min at room temperature or at 30 °C.
- Centrifuge for 5 min, 8500 rpm, and discard supernatant.
- Wash the pellet with 500 µL of ethanol (100%) by vortex. Repeat step 9.
- Dry pellets at room temperature or at 60 °C.
- Resuspend in 40 µL miliQ water.
June 26, 2013
- We made competent yeast following the procedure mentioned before.
- We also made our first PCRs! We used primers 6 & 1 (A) and 34 & 9 (B) to extract VP16 and GCR from the Nal1 plasmid.
- Confirmation gel (2013-06-26 19 hr 16 min.jpg & 2013-06-26 19hr 15 min.jpg) with wells:
- Ladder
- PCR A
- PCR B
- Miniprep for Nal. 1
A = VP16
B = GCR
June 27, 2013
We repeated the PCR for A and used lambda phage DNA for carrier DNA. We also tried extracting yeast genome using a modified Harju “Bust n’ Grab” protocol using three parallel methods:
- Method “H” used the regular lysis buffer.
- Method “C” used the following lysis buffer: 3% Triton, 100 mM LiCl, 10 mM Tris-HCl, 1 mM EDTA, 100 mM NaAc.
- Method “O” used the following lysis buffer: 2% triton, 1& SDS, 10 mM tris-HCl, 1 mM EDTA, 100 mM LiCl.
Conformation gel was run with wells:
- Ladder
- PCR A (repeated)
- Carrier lambda PCR
- Method C
- Method H
- Method O
The genome extraction still isn't working! :(
We then ran a fusion PCR with GAL4 and VP16 (A and B). These were the PCR conditions:
- 1st PCR --> 2-step PCR
Cycle steps- Initial denaturation (98 °C, 30 s)
- 15 cycles (98 °C, 10 s; 72 °C, 35 s)
- Final extension (72 °C, 5 min)
- Hold (4 °C, indefinite time)
- 2nd PCR --> 2-step PCR (add primers)
Cycle steps- Initial denaturation (98 °C, 30 s)
- 35 cycles (98 °C, 10 s; 72 °C, 35 s)
- Final extension (72 °C, 10 min)
- Hold (4 °C, indefinite time)
- Solution I: Glucose 50 mM, EDTA pH 8 10 mM, Tris-HCl pH 8 25 mM (esterilized) + Zymolyase
- Solution II: NaOH 0.2 N, SPS 1%
- A: Zymolyase for ½ h at 37 °C
- B: Zymolyase for ½ h at 37 °C, then protease K + lysis T ½ h at 55 °C
- C: Zymolyase for ½ h at 37 °C, then protease K + lysis T ½ h at 37 °C
- D: Regular GenElute Genome extraction protocol
- 98 °C, 1 min
- 98 °C, 10 s
- 72 °C, 45 s
- 72 °C, 10 min
- 4 °C
- pBap2 = C
- GAL4 = D
- VP16 = A
- GCR = B
- TER = F
- VP16 + GCR = AB
- pBAP2 + GAL4 = CD
- AB + TER = ABF
- Terminator (114486764) (F1 & F2)
- 3X UAS-pGAL1 (114486765) (E2)
- 6X UAS-pGAL1 (114486766) (E3)
- 9X UAS-pGAL1 (114486767) (E4)
July 2nd, 2013
Still trying to successfully extract the yeast genome! This time we tried an alternate method where we used two different solutions to break the cell wall:
The rest of the protocol was taken from GenElute DNA Kit from Sigma-Aldrich.
July 3rd, 2013
The genome extraction was better, but it's mostly degraded DNA! We still have to improve the protocol.
July 5th, 2013
We're still improving our genome extraction protocol. This time we're trying 4 variations to break the cell wall
We're varying the incubation of both solutions, the one that comes with the kit (Proteinase K + Lysis buffer) and the zymolyase solution we previously used. The four variations were as follows.
The rest of the steps were done following the instructions from the GenElute Genome extraction protocol.
We ran a gel in this order: WM, A, B, C, D.
Both B and C gave results, with C giving better yields! We're keeping the C protocol and we're happy we can start extracting parts from the yeast genome!
We performed PCRs for BAP2, GAL4, yeast terminator and pGAL1. These were the conditions:
PCR fusion 2 steps*, with process 3 at 72 °C for 45 s
- *
Steps 2 and 3 x 35
July 9th, 2013
Today we ran PCRs for pGAL1 and mCherry. Now that we have several parts, we must have a clear understanding of our notation!
July 18th, 2013
Today we ran PCRs for the following fusions: F1 = TER-GCR, with primers 31 & 35; F2 = TER – mCherry, with primers 31 & 33. We also amplified E = pGAL1, using primers 13 & 15.
July 19th, 2013
Today we amplified F2 that we obtained yesterday.
July 22nd, 2013
Today we tried to fuse C-D using primers 19 and 5, TER-GCR using primers 31 and 35 and TER-mCherry using primers 31 and 33. After 15 PCR cycles, we added the primers.
July 24th, 2013
Today we extracted the terminator from the yeast genome (F1 and F2) and pGAL1 from miniprep (E). We also did PCRs to fuse again our big parts: pBAP2 – GAL4 – VP16 – GCR (CD-AB) (primers 19 & 34) and pGAL1 – mCherry (E-G) (primers 32 & 15).
At the moment, this is our progress!:
August 5th, 2013
Things are advancing at a fast pace! Today we did miniprep to obtain PUC19!
August 6th, 2013
After doing a confirmation gel, C and CD are no longer with us! So we need to repeat them!
August 16th, 2013
Still no C nor D, so we did PCRs again with different methods to obtain C1, C2, D1 and D2.
September 3rd, 2013
Today we confirmed what we will be sending for the regional competition!
qBlocks (200 ng):
Final volume: (40 µL)
Final concentration: 5 ng/µL
In our quest to obtain again all our parts, we performed the following fusion PCRs: ABF1 (primers 1 & 31), GF2 (primers 17 & 31), E1GF2 (primers 15 & 31), E2G (primers 15 & 13), E3G (primers 15 & 13), E4G (primers 15 & 13).
We also performed the transformation by TOPO cloning.
September 4th, 2013
<p align="justify"> We inoculated cells with dexamethasone. The original syringe had an initial concentration of 8mg/2mL. We obtained an initial volume of 3.189*10^-2 µL, so we had to dilute first the dexamethasone: 9900 µL water + 100 µL dexametasona.</p>
<p align="justify">Induction experiment ON: 5 mL LB + 20 µL ampicillin + 20 µL kanamycin + 10 µL inoculum + 32 µL dexamethasone (1:1000) (inoculate ON).</p>
<p align="justify"> Gel PCR: F-H-P-E1GF2-MP-E2GF-E3GF1-E3GF2-E4GF- Everything was succesful except E1GF2.</p> <p align="justify"> We digested the obtained PCRs with 5 µL Buffer CutSmart + 15 µL PCR +1 µL XbaI + 1 µL SpeI + 28 µL water (for each PCR).</p>
 "
"