Template:Kyoto/Notebook/Sep 8
From 2013.igem.org
(Difference between revisions)
(→PCR) |
(→Ligation) |
||
| (25 intermediate revisions not shown) | |||
| Line 16: | Line 16: | ||
|6||RBS-lysis3-DT||XbaI & PstI | |6||RBS-lysis3-DT||XbaI & PstI | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_981b.png]]<br> |
| - | [[File: | + | [[File:igku_981a.png]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Name||concentration[µg/mL]||260/280||260/230 | !Name||concentration[µg/mL]||260/280||260/230 | ||
| Line 42: | Line 42: | ||
|10||RBS-lacZα-DT||XbaI & PstI | |10||RBS-lacZα-DT||XbaI & PstI | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_982b.png]]<br> |
| - | [[File: | + | [[File:igku_982a.png]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Name||concentration[µg/mL]||260/280||260/230 | !Name||concentration[µg/mL]||260/280||260/230 | ||
| Line 66: | Line 66: | ||
|6||pSB4K5||EcoRI & SpeI | |6||pSB4K5||EcoRI & SpeI | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_983b.png]]<br> |
| - | [[File: | + | [[File:igku_983a.png]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Name||concentration[µg/mL]||260/280||260/230 | !Name||concentration[µg/mL]||260/280||260/230 | ||
| Line 96: | Line 96: | ||
|12||pSB1C3||XbaI & PstI | |12||pSB1C3||XbaI & PstI | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_984b.png]]<br> |
| - | [[File: | + | [[File:igku_984a.png]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Name||concentration[µg/mL]||260/280||260/230 | !Name||concentration[µg/mL]||260/280||260/230 | ||
| Line 120: | Line 120: | ||
|Pcon-Spinach-DT||253.7||1.57||1.51 | |Pcon-Spinach-DT||253.7||1.57||1.51 | ||
|} | |} | ||
| + | ===Ligation=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Kojima</span> | ||
| + | {| class="wikitable" | ||
| + | !state||colspan="2"|Vector(µL)||colspan="2"|Inserter(µL)||Ligation High ver.2 | ||
| + | |- | ||
| + | |experiment||9/8 Plac SpeI+PstI||4.3||9/3 pT181 attenuator||3.1||3.5 | ||
| + | |- | ||
| + | |experiment||9/8 Plac SpeI+PstI||4.3||9/7 aptamer12-1R-DT XbaI+PstI||2.1||3.2 | ||
| + | |- | ||
| + | |experiment||9/8 Plac SpeI+PstI||4.3||9/8 spinach-DT XbaI+PstI||4.6||3.5 | ||
| + | |- | ||
| + | |experiment||9/6 Plac SpeI+PstI||2||9/7 RBS-lysis2-DT XbaI+PstI||10||3.5 | ||
| + | |- | ||
| + | |experiment||9/8 Plac SpeI+PstI||4.3||9/7 RBS-lysis2-DT XbaI+PstI||10||3.5 | ||
| + | |- | ||
| + | |experiment||9/8 Ptet-pT181 anti||1.6||9/8 spinach-DT XbaI+PstI||4.3||3.0 | ||
| + | |- | ||
| + | |experiment||9/6 Pcon SpeI+PstI||1.4||9/8 RBS-laczα-DT XbaI+PstI||4.8||3.1 | ||
| + | |- | ||
| + | |experiment||9/8 Pcon-pT181 attenuator SpeI+PstI||3.4||9/7 aptamer12-1R-DT XbaI+PstI||1.9||2.7 | ||
| + | |- | ||
| + | |experiment||9/6 Pcon SpeI+PstI||1.4||9/7 aptamer12-1R-DT XbaI+PstI||2.1||1.3 | ||
| + | |- | ||
| + | |experiment||9/8 DT||3.0||9/8 Pcon-pT181 attenuator EcoRI+SpeI||7.9||3.5 | ||
| + | |- | ||
| + | |experiment||9/6 Plux SpeI+PstI||2||9/7 RBS-lysis1-DT XbaI+PstI||7.2||3.5 | ||
| + | |- | ||
| + | |experiment||9/8 Plac SpeI+PstI||4.3||9/7 RBS-lysis1-DT XbaI+PstI||7.2||3.5 | ||
| + | |- | ||
| + | |experiment||9/4 Plac SpeI+PstI||1.7||9/8 RBS-laczα-DT XbaI+PstI||4.8||3.3 | ||
| + | |} | ||
| + | </div> | ||
===Restriction Enzyme Digestion=== | ===Restriction Enzyme Digestion=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">Kojima</span> |
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||9/8 Pcon-pT181 anti|| | + | ! ||9/8 Pcon-pT181 anti||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts||8.2µL|| 0µL|| 1µL|| 0µL|| 1µL|| 3µL|| 3µL|| 13.8µL|| 30µL | |2 cuts||8.2µL|| 0µL|| 1µL|| 0µL|| 1µL|| 3µL|| 3µL|| 13.8µL|| 30µL | ||
| Line 133: | Line 165: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||9/8 Pcon- | + | ! ||9/8 Pcon-spinach-DT||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts||7.9µL|| 1µL|| 1µL|| 0µL|| 0µL|| 3µL|| 3µL||14.1µL|| 30µL | |2 cuts||7.9µL|| 1µL|| 1µL|| 0µL|| 0µL|| 3µL|| 3µL||14.1µL|| 30µL | ||
| Line 140: | Line 172: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||8/21 F1 | + | ! ||8/21 F1 attenuator||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts|| 6.5µL|| 0µL|| 0µL|| 1µL|| 1µL|| 3µL|| 3µL||15.5µL||30µL | |2 cuts|| 6.5µL|| 0µL|| 0µL|| 1µL|| 1µL|| 3µL|| 3µL||15.5µL||30µL | ||
| Line 147: | Line 179: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||8/21 F3m2 | + | ! ||8/21 F3m2 attenuator||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts||6.0µL||0µL||0µL||1µL||1µL||3µL||3µL||16µL||30µL | |2 cuts||6.0µL||0µL||0µL||1µL||1µL||3µL||3µL||16µL||30µL | ||
| Line 154: | Line 186: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||8/21 F1 anti|| | + | ! ||8/21 F1 anti||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts||6.7µL||0µL||0µL||1µL||1µL||3µL||3µL||15.3µL||30µL | |2 cuts||6.7µL||0µL||0µL||1µL||1µL||3µL||3µL||15.3µL||30µL | ||
| Line 161: | Line 193: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||8/21 F6 anti|| | + | ! ||8/21 F6 anti||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts||6.0µL||0µL||0µL||1µL||1µL||3µL||3µL||16µL||30µL | |2 cuts||6.0µL||0µL||0µL||1µL||1µL||3µL||3µL||16µL||30µL | ||
| Line 168: | Line 200: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||8/21 tetR aptamer 12_1M|| | + | ! ||8/21 tetR aptamer 12_1M||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts||5.3µL||1µL||1µL||0µL||0µL||3µL||3µL||16.7µL||30µL | |2 cuts||5.3µL||1µL||1µL||0µL||0µL||3µL||3µL||16.7µL||30µL | ||
| Line 175: | Line 207: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||8/21 tetR aptamer 12_P|| | + | ! ||8/21 tetR aptamer 12_P||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts||13.3µL||1µL||1µL||0µL||0µL||3µL||3µL||8.7µL||30µL | |2 cuts||13.3µL||1µL||1µL||0µL||0µL||3µL||3µL||8.7µL||30µL | ||
| Line 182: | Line 214: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||9/4 | + | ! ||9/4 spinach-DT||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
||2 cuts||5.4µL||0µL||0µL||1µL||1µL||3µL||3µL||16.6µL||30µL | ||2 cuts||5.4µL||0µL||0µL||1µL||1µL||3µL||3µL||16.6µL||30µL | ||
| Line 189: | Line 221: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||9/7 RBS-lysis1-DT|| | + | ! ||9/7 RBS-lysis1-DT||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts|||10µL||0µL||0µL||1µL||1µL||3µL||3µL||12µL||30µL | |2 cuts|||10µL||0µL||0µL||1µL||1µL||3µL||3µL||12µL||30µL | ||
| Line 196: | Line 228: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||9/6 RBS-lysis2-DT|| | + | ! ||9/6 RBS-lysis2-DT||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
||2 cuts||13µL||0µL||0µL||1µL||1µL||3µL||3µL||9µL||30µL | ||2 cuts||13µL||0µL||0µL||1µL||1µL||3µL||3µL||9µL||30µL | ||
| Line 203: | Line 235: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
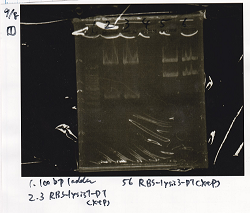
| - | ! ||9/7 RBS-lysis3-DT|| | + | ! ||9/7 RBS-lysis3-DT||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts|||10µL||0µL||0µL||1µL||1µL||3µL||3µL||12µL||30µL | |2 cuts|||10µL||0µL||0µL||1µL||1µL||3µL||3µL||12µL||30µL | ||
|- | |- | ||
|NC||0.5µL||0µL||0µL||0µL||0µL||1µL||1µL||7.5µL||10µL | |NC||0.5µL||0µL||0µL||0µL||0µL||1µL||1µL||7.5µL||10µL | ||
| - | |||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
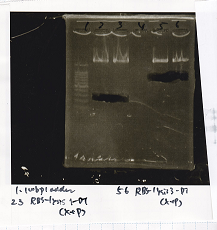
| - | ! ||8/20 J23100 RBS- | + | ! ||8/20 J23100 RBS-lacZα-DT-(1)||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts|||7µL||0.5µL||0.5µL||0µL||0µL||3µL||3µL||16µL||30µL | |2 cuts|||7µL||0.5µL||0.5µL||0µL||0µL||3µL||3µL||16µL||30µL | ||
|- | |- | ||
|NC||0.5µL||0µL||0µL||0µL||0µL||1µL||1µL||7.5µL||10µL | |NC||0.5µL||0µL||0µL||0µL||0µL||1µL||1µL||7.5µL||10µL | ||
| - | |||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||8/20 RBS- | + | ! ||8/20 RBS-lacZα-DT||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts||10µL||0.5µL||0.5µL||0µL||0µL||3µL||3µL||13µL||30µL | |2 cuts||10µL||0.5µL||0.5µL||0µL||0µL||3µL||3µL||13µL||30µL | ||
|- | |- | ||
|NC||0.5µL||0µL||0µL||0µL||0µL||1µL||1µL||7.5µL||10µL | |NC||0.5µL||0µL||0µL||0µL||0µL||1µL||1µL||7.5µL||10µL | ||
| - | |||
|} | |} | ||
| + | </div> | ||
===Transformation=== | ===Transformation=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">Nakamoto & Ashida</span> |
{| class="wikitable" | {| class="wikitable" | ||
!Name||Sample(µL)||Competent Cells(µL)||Total(µL)||Plate | !Name||Sample(µL)||Competent Cells(µL)||Total(µL)||Plate | ||
| Line 236: | Line 265: | ||
|9/8 Pcont+aptamer 12-1R-DT||1||10||11||Amp | |9/8 Pcont+aptamer 12-1R-DT||1||10||11||Amp | ||
|- | |- | ||
| - | |9/8 Pcon+RBS- | + | |9/8 Pcon+RBS-lacZα-DT||1||10||11||Amp |
|- | |- | ||
| - | |9/8 Pcon-pT181 | + | |9/8 Pcon-pT181 attenuator+aptamer 12-1R-DT||1||10||11||Amp |
|- | |- | ||
|9/8 Plac+RBS-lysis1-DT||2||20||22||CP | |9/8 Plac+RBS-lysis1-DT||2||20||22||CP | ||
| Line 248: | Line 277: | ||
|9/8 Plac+spinach||2||20||22||CP | |9/8 Plac+spinach||2||20||22||CP | ||
|- | |- | ||
| - | |9/8 Plac+pT181 | + | |9/8 Plac+pT181 attenuator||2||20||22||CP |
|- | |- | ||
|9/8 Plux+RBS-lysis1-DT||1||10||11||CP | |9/8 Plux+RBS-lysis1-DT||1||10||11||CP | ||
| Line 254: | Line 283: | ||
|9/8 Plux+RBS-lysis2-DT||1||10||11||CP | |9/8 Plux+RBS-lysis2-DT||1||10||11||CP | ||
|- | |- | ||
| - | |9/8 Ptet+RBS- | + | |9/8 Ptet+RBS-lacZα-DT||1||10||11||CP |
|- | |- | ||
|9/8 Ptet-pT181 anti+spinach-DT||1||10||11||CP | |9/8 Ptet-pT181 anti+spinach-DT||1||10||11||CP | ||
|- | |- | ||
| - | |9/8 Pcon-pT181 | + | |9/8 Pcon-pT181 attenuator+DT||1||10||11||CP |
|} | |} | ||
| + | </div> | ||
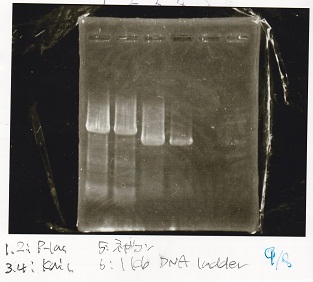
===Electrophoresis=== | ===Electrophoresis=== | ||
| - | |||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">No name</span> |
{| class="wikitable" | {| class="wikitable" | ||
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
| Line 271: | Line 299: | ||
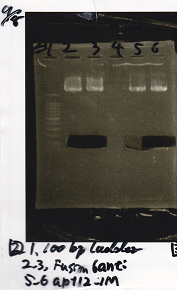
|1||100bp ladder|| || | |1||100bp ladder|| || | ||
|- | |- | ||
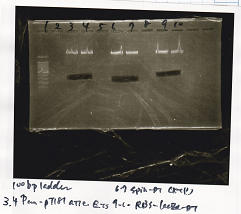
| - | |2||Pcon- | + | |2||Pcon-spinach-DT||EcoRI||SpeI |
|- | |- | ||
| - | |3||Pcon- | + | |3||Pcon-spinach-DT|| || |
|- | |- | ||
| - | |4||Fusion1 attenuator|| | + | |4||Fusion1 attenuator||XbaI||PstI |
|- | |- | ||
|5||Fusion1 attenuator|| || | |5||Fusion1 attenuator|| || | ||
|- | |- | ||
| - | |6||Fusion3m2 attenuator|| | + | |6||Fusion3m2 attenuator||XbaI||PstI |
|- | |- | ||
|7||Fusion3m2 attenuator|| || | |7||Fusion3m2 attenuator|| || | ||
|- | |- | ||
| - | |8||Fusion1 anti|| | + | |8||Fusion1 anti||XbaI||PstI |
|- | |- | ||
|9||Fusion1 anti|| || | |9||Fusion1 anti|| || | ||
|- | |- | ||
| - | |10||Fusion6 anti|| | + | |10||Fusion6 anti||XbaI||PstI |
|- | |- | ||
|11||Fusion6 anti|| || | |11||Fusion6 anti|| || | ||
|- | |- | ||
| - | |12||aptamer 12-1M|| | + | |12||aptamer 12-1M||XbaI||PstI |
|- | |- | ||
|13||aptamer 12-1M|| || | |13||aptamer 12-1M|| || | ||
|- | |- | ||
| - | |14||aptamer 12-P|| | + | |14||aptamer 12-P||XbaI||PstI |
|- | |- | ||
|15||aptamer 12-P|| || | |15||aptamer 12-P|| || | ||
|- | |- | ||
| - | |16||spinach-DT|| | + | |16||spinach-DT||XbaI||PstI |
|- | |- | ||
|} | |} | ||
| + | [[File:Igku_985.png]] | ||
{| class="wikitable" | {| class="wikitable" | ||
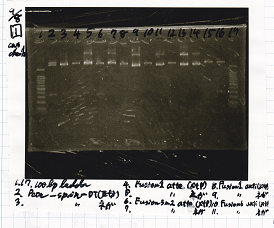
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
| Line 307: | Line 336: | ||
|1||spinach-DT|| || | |1||spinach-DT|| || | ||
|- | |- | ||
| - | |2||J23100-RBS- | + | |2||J23100-RBS-lacZα-DT||EcoRI||SpeI |
|- | |- | ||
| - | |3||J23100-RBS- | + | |3||J23100-RBS-lacZα-DT|| || |
|- | |- | ||
|4||100bp ladder|| || | |4||100bp ladder|| || | ||
|- | |- | ||
| - | |5||RBS- | + | |5||RBS-lacZα-DT||EcoRI||SpeI |
|- | |- | ||
| - | |6||RBS- | + | |6||RBS-lacZα-DT|| || |
|- | |- | ||
| - | |7||RBS-lysis1-DT|| | + | |7||RBS-lysis1-DT||XbaI||PstI |
|- | |- | ||
|8||RBS-lysis1-DT|| || | |8||RBS-lysis1-DT|| || | ||
| Line 327: | Line 356: | ||
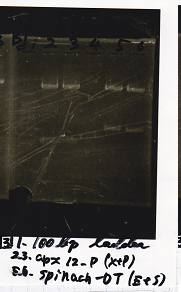
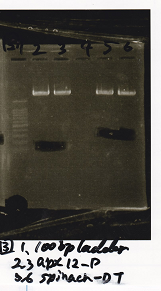
|1||100bp ladder|| || | |1||100bp ladder|| || | ||
|- | |- | ||
| - | |2||RBS-lysis2-DT|| | + | |2||RBS-lysis2-DT||XbaI||PstI |
|- | |- | ||
|3||RBS-lysis2-DT|| || | |3||RBS-lysis2-DT|| || | ||
|- | |- | ||
| - | |4||RBS-lysis3-DT|| | + | |4||RBS-lysis3-DT||XbaI||PstI |
|- | |- | ||
|5||RBS-lysis3-DT|| || | |5||RBS-lysis3-DT|| || | ||
|- | |- | ||
| - | |6||Pcon pT181 anti|| | + | |6||Pcon pT181 anti||SpeI||PstI |
|- | |- | ||
|7||Pcon pT181 anti|| || | |7||Pcon pT181 anti|| || | ||
|- | |- | ||
|} | |} | ||
| + | [[File:Igku_986.png]] | ||
| + | </div> | ||
===Gel Extraction=== | ===Gel Extraction=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">Ashida and Nakamoto</span> |
{| class="wikitable" | {| class="wikitable" | ||
!Lane||DNA||Enzyme | !Lane||DNA||Enzyme | ||
| Line 350: | Line 380: | ||
|1||100bp ladder|| | |1||100bp ladder|| | ||
|- | |- | ||
| - | |2||Pcon- | + | |2||Pcon-spinach-DT||EcoRI+SpeI |
|- | |- | ||
| - | |3||Pcon- | + | |3||Pcon-Spinach-DT||EcoRI+SpeI |
|- | |- | ||
| - | |5||Fusion 1 attenuator|| | + | |5||Fusion 1 attenuator||XbaI+PstI |
|- | |- | ||
| - | |6||Fusion 1 attenuator|| | + | |6||Fusion 1 attenuator||XbaI+PstI |
|- | |- | ||
| - | |8||Fusion3m2 attenuator|| | + | |8||Fusion3m2 attenuator||XbaI+PstI |
|- | |- | ||
| - | |9||Fusion3m2 attenuator|| | + | |9||Fusion3m2 attenuator||XbaI+PstI |
|- | |- | ||
| - | |11||Fusion 1 | + | |11||Fusion 1 antisense||XbaI+PstI |
|- | |- | ||
| - | |12||Fusion 1 | + | |12||Fusion 1 antisense||XbaI+PstI |
|- | |- | ||
|} | |} | ||
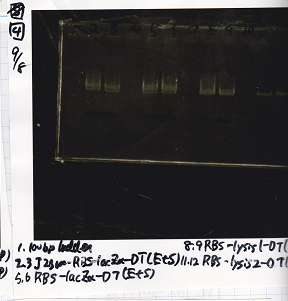
| - | [[File: | + | [[File:igku_987.png]]<br> |
| - | + | ||
{| class="wikitable" | {| class="wikitable" | ||
!Name||quantity||concentration[µg/mL]||260/280||260/230 | !Name||quantity||concentration[µg/mL]||260/280||260/230 | ||
|- | |- | ||
| - | |Pcon- | + | |Pcon-spinach-DT(EcoRI+SpeI)|| ||10.5||1.86||0.39 |
|- | |- | ||
| - | |Fusion 1 attenuator( | + | |Fusion 1 attenuator(XbaI+PstI)|| ||7.0||1.71||0.27 |
|- | |- | ||
| - | |Fusion3m2 attenuator( | + | |Fusion3m2 attenuator(XbaI+PstI)|| ||8.6||1.75||0.47 |
|- | |- | ||
| - | |Fusion 1 | + | |Fusion 1 antisense(XbaI+PstI)|| ||7.6||1.87||0.43 |
|- | |- | ||
|} | |} | ||
| Line 386: | Line 415: | ||
|1||100bp ladder|| | |1||100bp ladder|| | ||
|- | |- | ||
| - | |2||Fusion6 | + | |2||Fusion6 antisense||XbaI+PstI |
|- | |- | ||
| - | |3||Fusion6 | + | |3||Fusion6 antisense||XbaI+PstI |
|- | |- | ||
| - | |5||aptamer12_1M|| | + | |5||aptamer12_1M||XbaI+PstI |
|- | |- | ||
| - | |6||aptamer12_1M|| | + | |6||aptamer12_1M||XbaI+PstI |
|- | |- | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_988b.png]]<br> |
| - | [[File: | + | [[File:igku_988a.png]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Name||quantity||concentration[µg/mL]||260/280||260/230 | !Name||quantity||concentration[µg/mL]||260/280||260/230 | ||
|- | |- | ||
| - | |Fusion6 | + | |Fusion6 antisense(XbaI+PstI)|| ||5.0||1.76||0.20 |
|- | |- | ||
| - | |aptamer12_1M( | + | |aptamer12_1M(XbaI+PstI)|| ||3.3||2.31||0.04 |
|- | |- | ||
|} | |} | ||
| Line 410: | Line 439: | ||
|1||100bp ladder|| | |1||100bp ladder|| | ||
|- | |- | ||
| - | |2||aptamer12_P|| | + | |2||aptamer12_P||XbaI+PstI |
|- | |- | ||
| - | |3||aptamer12_P|| | + | |3||aptamer12_P||XbaI+PstI |
|- | |- | ||
| - | |5||spinach-DT|| | + | |5||spinach-DT||EcoRI+SpeI |
|- | |- | ||
| - | |6||spinach-DT|| | + | |6||spinach-DT||EcoRI+SpeI |
|- | |- | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_989b.png]]<br> |
| - | [[File: | + | [[File:igku_989a.png]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Name||quantity||concentration[µg/mL]||260/280||260/230 | !Name||quantity||concentration[µg/mL]||260/280||260/230 | ||
|- | |- | ||
| - | |aptamer12_P( | + | |aptamer12_P(XbaI+PstI)|| ||3.4||1.72||0.26 |
|- | |- | ||
| - | |spinach-DT( | + | |spinach-DT(EcoRI+SpeI)|| ||10.1||1.57||0.47 |
|- | |- | ||
|} | |} | ||
| Line 434: | Line 463: | ||
|1||100bp ladder|| | |1||100bp ladder|| | ||
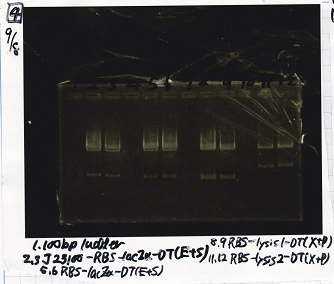
|- | |- | ||
| - | |2||J23100-RBS- | + | |2||J23100-RBS-laczα-DT||EcoRI+SpeI |
|- | |- | ||
| - | |3||J23100-RBS- | + | |3||J23100-RBS-laczα-DT||EcoRI+SpeI |
|- | |- | ||
| - | |5||RBS- | + | |5||RBS-laczα-DT||EcoRI+SpeI |
|- | |- | ||
| - | |6||RBS- | + | |6||RBS-laczα-DT||EcoRI+SpeI |
|- | |- | ||
| - | |8||RBS-lysis1-DT|| | + | |8||RBS-lysis1-DT||XbaI+PstI |
|- | |- | ||
| - | |9||RBS-lysis1-DT|| | + | |9||RBS-lysis1-DT||XbaI+PstI |
|- | |- | ||
| - | |11||RBS-lysis2-DT|| | + | |11||RBS-lysis2-DT||XbaI+PstI |
|- | |- | ||
| - | |12||RBS-lysis2-DT|| | + | |12||RBS-lysis2-DT||XbaI+PstI |
|- | |- | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_9810b.png]]<br> |
| - | [[File: | + | [[File:igku_9810a.png]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Name||quantity||concentration[µg/mL]||260/280||260/230 | !Name||quantity||concentration[µg/mL]||260/280||260/230 | ||
|- | |- | ||
| - | |Pcon-RBS- | + | |Pcon-RBS-laczα-DT(EcoRI+SpeI)|| ||5.6||2.03||0.25 |
|- | |- | ||
| - | |RBS- | + | |RBS-laczα-DT(EcoRI+SpeI)|| ||4.4||2.04||0.33 |
|- | |- | ||
| - | |RBS-lysis1-DT( | + | |RBS-lysis1-DT(XbaI+PstI)|| ||5.9||2.22||0.53 |
|- | |- | ||
| - | |RBS-lysis2-DT( | + | |RBS-lysis2-DT(XbaI+PstI)|| ||5.1||1.67||0.48 |
|- | |- | ||
|} | |} | ||
| Line 470: | Line 499: | ||
|1||100bp ladder|| | |1||100bp ladder|| | ||
|- | |- | ||
| - | |2||RBS-lysis3-DT|| | + | |2||RBS-lysis3-DT||XbaI+PstI |
|- | |- | ||
| - | |3||RBS-lysis3-DT|| | + | |3||RBS-lysis3-DT||XbaI+PstI |
|- | |- | ||
| - | |5||Pcon-pT181 | + | |5||Pcon-pT181 antisense||SpeI+PstI |
|- | |- | ||
| - | |6||Pcon-pT181 | + | |6||Pcon-pT181 antisense||SpeI+PstI |
|- | |- | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_9811b.png]]<br> |
| - | [[File: | + | [[File:igku_9811a.png]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Name||quantity||concentration[µg/mL]||260/280||260/230 | !Name||quantity||concentration[µg/mL]||260/280||260/230 | ||
|- | |- | ||
| - | |RBS-lysis3-DT( | + | |RBS-lysis3-DT(XbaI+PstI)|| ||23.4||1.72||0.88 |
|- | |- | ||
| - | |Pcon-pT181 | + | |Pcon-pT181 antisense(SpeI+PstI)|| ||31.3||1.86||0.92 |
|- | |- | ||
|} | |} | ||
| + | </div> | ||
===Ligation=== | ===Ligation=== | ||
| - | |||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">No name</span> |
{| class="wikitable" | {| class="wikitable" | ||
| - | !state||colspan="2"|Vector||colspan="2"|Inserter | + | !state||colspan="2"|Vector||colspan="2"|Inserter |
|- | |- | ||
| - | |experiment|| | + | |experiment||Pcon-spinach-DT(EcoRI&SpeI)||10.5µg/mL||pSB6A1(EcoRI&SpeI)||8.9µg/mL |
|- | |- | ||
| - | | | + | |experiment||Pcon-spinach-DT(EcoRI&SpeI)||10.5µg/mL||pSB4K5(EcoRI&SpeI)||21.8µg/mL |
| - | + | ||
| - | + | ||
| - | + | ||
|- | |- | ||
| - | |experiment|| | + | |experiment||Pcon-pT181 anti(SpeI+PstI)||31.3µg/mL||spinach-DT(XbaI+PstI)||10.1µg/mL |
| + | |- | ||
| + | |experiment||Plac(SpeI+PstI)||11.6µg/mL||RBS-lysis3-DT||23.4µg/mL | ||
| + | |- | ||
| + | |experiment||Plux(SpeI+PstI)||25µg/mL||RBS-lysis3-DT||23.4µg/mL | ||
| + | |- | ||
| + | |experiment||Ptet-pT181 anti(SpeI+PstI)||30.6µg/mL||spinach-DT(XbaI+PstI)||10.1µg/mL | ||
| + | |- | ||
| + | |experiment||Pcon-pT181 attenuator(SpeI+PstI)||14.5µg/mL||RBS-laczα-DT(XbaI+PstI)||4.4µg/mL | ||
|- | |- | ||
| - | |||
|} | |} | ||
| + | </div> | ||
| + | |||
===PCR=== | ===PCR=== | ||
<!-- ここから --> | <!-- ここから --> | ||
| Line 587: | Line 621: | ||
===Gel Extraction=== | ===Gel Extraction=== | ||
| - | |||
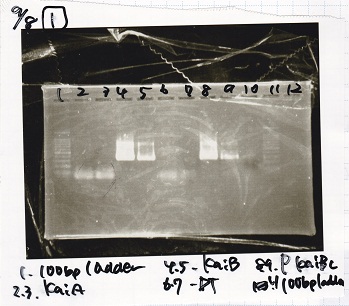
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">Yoshida</span> |
{| class="wikitable" | {| class="wikitable" | ||
| - | !Lane||DNA | + | !Lane||DNA |
|- | |- | ||
| - | | | + | |1||100bp ladder |
|- | |- | ||
| - | | || | + | |2||KaiA |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|- | |- | ||
| - | | | + | |3||KaiA |
|- | |- | ||
| - | | | + | |4||KaiB |
| - | + | ||
| - | + | ||
| - | + | ||
|- | |- | ||
| - | | | + | |5||KaiB |
| + | |- | ||
| + | |6||DT | ||
| + | |- | ||
| + | |7||DT | ||
| + | |- | ||
| + | |8||P-KaiBC | ||
| + | |- | ||
| + | |9||P-KaiBC | ||
| + | |- | ||
| + | |10||nega | ||
| + | |- | ||
| + | |11||100bp ladder | ||
|- | |- | ||
| - | |||
|} | |} | ||
| - | [[File: | + | [[File:9898.jpg]]<br> |
| - | [[File: | + | [[File:9897.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
| - | ! | + | !Lane||DNA |
| + | |- | ||
| + | |1||Plac | ||
| + | |- | ||
| + | |2||Plac | ||
| + | |- | ||
| + | |3||KaiC | ||
| + | |- | ||
| + | |4||KaiC | ||
| + | |- | ||
| + | |5||nega | ||
|- | |- | ||
| - | | | + | |6||1kb ladder |
|- | |- | ||
| - | |||
|} | |} | ||
| + | [[File:9899.jpg]]<br> | ||
| + | [[File:991.jpg]]<br> | ||
| + | </div> | ||
Latest revision as of 14:25, 27 September 2013
Contents |
Sep 8
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | |
| 2 | RBS-lysis1-DT | XbaI & PstI |
| 3 | RBS-lysis1-DT | XbaI & PstI |
| 5 | RBS-lysis3-DT | XbaI & PstI |
| 6 | RBS-lysis3-DT | XbaI & PstI |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| RBS-lysis1-DT (XbaI & PstI) | 5.3 | 1.98 | 0.06 |
| RBS-lysis3-DT (XbaI & PstI) | 5.3 | 1.98 | 0.06 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | |
| 3 | Pcon-pT181 attenuator | EcoRI & SpeI |
| 4 | Pcon-pT181 attenuator | EcoRI & SpeI |
| 6 | Spinach-DT | XbaI & PstI |
| 7 | Spinach-DT | XbaI & PstI |
| 9 | RBS-lacZα-DT | XbaI & PstI |
| 10 | RBS-lacZα-DT | XbaI & PstI |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Pcon-pT181 attenuator (EcoRI & SpeI) | 5.2 | 1.82 | 0.36 |
| Spinach-DT (XbaI & PstI) | 8.0 | 1.87 | 0.25 |
| RBS-lacZα-DT (XbaI & PstI) | 10.3 | 1.71 | 0.54 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | |
| 2 | Ptet-pT181 antisense | EcoRI & PstI |
| 3 | Ptet-pT181 antisense | EcoRI & PstI |
| 5 | pSB4K5 | EcoRI & SpeI |
| 6 | pSB4K5 | EcoRI & SpeI |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Ptet-pT181 antisense(EcoRI & PstI) | 30.6 | 1.84 | 1.16 |
| pSB4K5 (EcoRI & SpeI) | 21.8 | 1.87 | 0.98 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | |
| 2 | Pcon-pT181 attenuator | SpeI & PstI |
| 3 | Pcon-pT181 attenuator | SpeI & PstI |
| 5 | Plac | SpeI & PstI |
| 6 | Plac | SpeI & PstI |
| 8 | pSB1C3 | EcoRI & SpeI |
| 9 | pSB1C3 | EcoRI & SpeI |
| 11 | pSB1C3 | XbaI & PstI |
| 12 | pSB1C3 | XbaI & PstI |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Pcon-pT181 attenuator (SpeI & PstI) | 14.5 | 1.91 | 1.09 |
| Plac (SpeI & PstI) | 11.6 | 1.74 | 1.19 |
| pSB1C3 (EcoRI & SpeI) | 5.7 | 1.74 | 0.34 |
| pSB1C3 (XbaI & PstI) | 5.6 | 2.00 | 0.37 |
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Pcon-pT181 anti | 243.0 | 1.90 | 1.81 |
| Pcon-Spinach-DT | 253.7 | 1.57 | 1.51 |
Ligation
| state | Vector(µL) | Inserter(µL) | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | 9/8 Plac SpeI+PstI | 4.3 | 9/3 pT181 attenuator | 3.1 | 3.5 |
| experiment | 9/8 Plac SpeI+PstI | 4.3 | 9/7 aptamer12-1R-DT XbaI+PstI | 2.1 | 3.2 |
| experiment | 9/8 Plac SpeI+PstI | 4.3 | 9/8 spinach-DT XbaI+PstI | 4.6 | 3.5 |
| experiment | 9/6 Plac SpeI+PstI | 2 | 9/7 RBS-lysis2-DT XbaI+PstI | 10 | 3.5 |
| experiment | 9/8 Plac SpeI+PstI | 4.3 | 9/7 RBS-lysis2-DT XbaI+PstI | 10 | 3.5 |
| experiment | 9/8 Ptet-pT181 anti | 1.6 | 9/8 spinach-DT XbaI+PstI | 4.3 | 3.0 |
| experiment | 9/6 Pcon SpeI+PstI | 1.4 | 9/8 RBS-laczα-DT XbaI+PstI | 4.8 | 3.1 |
| experiment | 9/8 Pcon-pT181 attenuator SpeI+PstI | 3.4 | 9/7 aptamer12-1R-DT XbaI+PstI | 1.9 | 2.7 |
| experiment | 9/6 Pcon SpeI+PstI | 1.4 | 9/7 aptamer12-1R-DT XbaI+PstI | 2.1 | 1.3 |
| experiment | 9/8 DT | 3.0 | 9/8 Pcon-pT181 attenuator EcoRI+SpeI | 7.9 | 3.5 |
| experiment | 9/6 Plux SpeI+PstI | 2 | 9/7 RBS-lysis1-DT XbaI+PstI | 7.2 | 3.5 |
| experiment | 9/8 Plac SpeI+PstI | 4.3 | 9/7 RBS-lysis1-DT XbaI+PstI | 7.2 | 3.5 |
| experiment | 9/4 Plac SpeI+PstI | 1.7 | 9/8 RBS-laczα-DT XbaI+PstI | 4.8 | 3.3 |
Restriction Enzyme Digestion
| 9/8 Pcon-pT181 anti | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 8.2µL | 0µL | 1µL | 0µL | 1µL | 3µL | 3µL | 13.8µL | 30µL |
| NC | 0.4µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.6µL | 10µL |
| 9/8 Pcon-spinach-DT | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 7.9µL | 1µL | 1µL | 0µL | 0µL | 3µL | 3µL | 14.1µL | 30µL |
| NC | 0.4µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.6µL | 10µL |
| 8/21 F1 attenuator | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 6.5µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 15.5µL | 30µL |
| NC | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL |
| 8/21 F3m2 attenuator | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 6.0µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 16µL | 30µL |
| NC | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL |
| 8/21 F1 anti | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 6.7µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 15.3µL | 30µL |
| NC | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL |
| 8/21 F6 anti | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 6.0µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 16µL | 30µL |
| NC | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL |
| 8/21 tetR aptamer 12_1M | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 5.3µL | 1µL | 1µL | 0µL | 0µL | 3µL | 3µL | 16.7µL | 30µL |
| NC | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL |
| 8/21 tetR aptamer 12_P | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 13.3µL | 1µL | 1µL | 0µL | 0µL | 3µL | 3µL | 8.7µL | 30µL |
| NC | 0.7µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.3µL | 10µL |
| 9/4 spinach-DT | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 5.4µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 16.6µL | 30µL |
| NC | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL |
| 9/7 RBS-lysis1-DT | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 10µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 12µL | 30µL |
| NC | 0.5µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL |
| 9/6 RBS-lysis2-DT | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 13µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 9µL | 30µL |
| NC | 0.7µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.3µL | 10µL |
| 9/7 RBS-lysis3-DT | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 10µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 12µL | 30µL |
| NC | 0.5µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL |
| 8/20 J23100 RBS-lacZα-DT-(1) | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 7µL | 0.5µL | 0.5µL | 0µL | 0µL | 3µL | 3µL | 16µL | 30µL |
| NC | 0.5µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL |
| 8/20 RBS-lacZα-DT | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 10µL | 0.5µL | 0.5µL | 0µL | 0µL | 3µL | 3µL | 13µL | 30µL |
| NC | 0.5µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL |
Transformation
| Name | Sample(µL) | Competent Cells(µL) | Total(µL) | Plate |
|---|---|---|---|---|
| 9/8 Pcont+aptamer 12-1R-DT | 1 | 10 | 11 | Amp |
| 9/8 Pcon+RBS-lacZα-DT | 1 | 10 | 11 | Amp |
| 9/8 Pcon-pT181 attenuator+aptamer 12-1R-DT | 1 | 10 | 11 | Amp |
| 9/8 Plac+RBS-lysis1-DT | 2 | 20 | 22 | CP |
| 9/8 Plac+RBS-lysis2-DT | 2 | 20 | 22 | CP |
| 9/8 Plac+aptamer 12-1R-DT | 2 | 20 | 22 | CP |
| 9/8 Plac+spinach | 2 | 20 | 22 | CP |
| 9/8 Plac+pT181 attenuator | 2 | 20 | 22 | CP |
| 9/8 Plux+RBS-lysis1-DT | 1 | 10 | 11 | CP |
| 9/8 Plux+RBS-lysis2-DT | 1 | 10 | 11 | CP |
| 9/8 Ptet+RBS-lacZα-DT | 1 | 10 | 11 | CP |
| 9/8 Ptet-pT181 anti+spinach-DT | 1 | 10 | 11 | CP |
| 9/8 Pcon-pT181 attenuator+DT | 1 | 10 | 11 | CP |
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | ||
| 2 | Pcon-spinach-DT | EcoRI | SpeI |
| 3 | Pcon-spinach-DT | ||
| 4 | Fusion1 attenuator | XbaI | PstI |
| 5 | Fusion1 attenuator | ||
| 6 | Fusion3m2 attenuator | XbaI | PstI |
| 7 | Fusion3m2 attenuator | ||
| 8 | Fusion1 anti | XbaI | PstI |
| 9 | Fusion1 anti | ||
| 10 | Fusion6 anti | XbaI | PstI |
| 11 | Fusion6 anti | ||
| 12 | aptamer 12-1M | XbaI | PstI |
| 13 | aptamer 12-1M | ||
| 14 | aptamer 12-P | XbaI | PstI |
| 15 | aptamer 12-P | ||
| 16 | spinach-DT | XbaI | PstI |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | spinach-DT | ||
| 2 | J23100-RBS-lacZα-DT | EcoRI | SpeI |
| 3 | J23100-RBS-lacZα-DT | ||
| 4 | 100bp ladder | ||
| 5 | RBS-lacZα-DT | EcoRI | SpeI |
| 6 | RBS-lacZα-DT | ||
| 7 | RBS-lysis1-DT | XbaI | PstI |
| 8 | RBS-lysis1-DT |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | ||
| 2 | RBS-lysis2-DT | XbaI | PstI |
| 3 | RBS-lysis2-DT | ||
| 4 | RBS-lysis3-DT | XbaI | PstI |
| 5 | RBS-lysis3-DT | ||
| 6 | Pcon pT181 anti | SpeI | PstI |
| 7 | Pcon pT181 anti |
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | |
| 2 | Pcon-spinach-DT | EcoRI+SpeI |
| 3 | Pcon-Spinach-DT | EcoRI+SpeI |
| 5 | Fusion 1 attenuator | XbaI+PstI |
| 6 | Fusion 1 attenuator | XbaI+PstI |
| 8 | Fusion3m2 attenuator | XbaI+PstI |
| 9 | Fusion3m2 attenuator | XbaI+PstI |
| 11 | Fusion 1 antisense | XbaI+PstI |
| 12 | Fusion 1 antisense | XbaI+PstI |
| Name | quantity | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|---|
| Pcon-spinach-DT(EcoRI+SpeI) | 10.5 | 1.86 | 0.39 | |
| Fusion 1 attenuator(XbaI+PstI) | 7.0 | 1.71 | 0.27 | |
| Fusion3m2 attenuator(XbaI+PstI) | 8.6 | 1.75 | 0.47 | |
| Fusion 1 antisense(XbaI+PstI) | 7.6 | 1.87 | 0.43 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | |
| 2 | Fusion6 antisense | XbaI+PstI |
| 3 | Fusion6 antisense | XbaI+PstI |
| 5 | aptamer12_1M | XbaI+PstI |
| 6 | aptamer12_1M | XbaI+PstI |
| Name | quantity | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|---|
| Fusion6 antisense(XbaI+PstI) | 5.0 | 1.76 | 0.20 | |
| aptamer12_1M(XbaI+PstI) | 3.3 | 2.31 | 0.04 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | |
| 2 | aptamer12_P | XbaI+PstI |
| 3 | aptamer12_P | XbaI+PstI |
| 5 | spinach-DT | EcoRI+SpeI |
| 6 | spinach-DT | EcoRI+SpeI |
| Name | quantity | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|---|
| aptamer12_P(XbaI+PstI) | 3.4 | 1.72 | 0.26 | |
| spinach-DT(EcoRI+SpeI) | 10.1 | 1.57 | 0.47 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | |
| 2 | J23100-RBS-laczα-DT | EcoRI+SpeI |
| 3 | J23100-RBS-laczα-DT | EcoRI+SpeI |
| 5 | RBS-laczα-DT | EcoRI+SpeI |
| 6 | RBS-laczα-DT | EcoRI+SpeI |
| 8 | RBS-lysis1-DT | XbaI+PstI |
| 9 | RBS-lysis1-DT | XbaI+PstI |
| 11 | RBS-lysis2-DT | XbaI+PstI |
| 12 | RBS-lysis2-DT | XbaI+PstI |
| Name | quantity | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|---|
| Pcon-RBS-laczα-DT(EcoRI+SpeI) | 5.6 | 2.03 | 0.25 | |
| RBS-laczα-DT(EcoRI+SpeI) | 4.4 | 2.04 | 0.33 | |
| RBS-lysis1-DT(XbaI+PstI) | 5.9 | 2.22 | 0.53 | |
| RBS-lysis2-DT(XbaI+PstI) | 5.1 | 1.67 | 0.48 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | |
| 2 | RBS-lysis3-DT | XbaI+PstI |
| 3 | RBS-lysis3-DT | XbaI+PstI |
| 5 | Pcon-pT181 antisense | SpeI+PstI |
| 6 | Pcon-pT181 antisense | SpeI+PstI |
| Name | quantity | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|---|
| RBS-lysis3-DT(XbaI+PstI) | 23.4 | 1.72 | 0.88 | |
| Pcon-pT181 antisense(SpeI+PstI) | 31.3 | 1.86 | 0.92 |
Ligation
| state | Vector | Inserter | ||
|---|---|---|---|---|
| experiment | Pcon-spinach-DT(EcoRI&SpeI) | 10.5µg/mL | pSB6A1(EcoRI&SpeI) | 8.9µg/mL |
| experiment | Pcon-spinach-DT(EcoRI&SpeI) | 10.5µg/mL | pSB4K5(EcoRI&SpeI) | 21.8µg/mL |
| experiment | Pcon-pT181 anti(SpeI+PstI) | 31.3µg/mL | spinach-DT(XbaI+PstI) | 10.1µg/mL |
| experiment | Plac(SpeI+PstI) | 11.6µg/mL | RBS-lysis3-DT | 23.4µg/mL |
| experiment | Plux(SpeI+PstI) | 25µg/mL | RBS-lysis3-DT | 23.4µg/mL |
| experiment | Ptet-pT181 anti(SpeI+PstI) | 30.6µg/mL | spinach-DT(XbaI+PstI) | 10.1µg/mL |
| experiment | Pcon-pT181 attenuator(SpeI+PstI) | 14.5µg/mL | RBS-laczα-DT(XbaI+PstI) | 4.4µg/mL |
PCR
| KaiA(plasmid) | KOD plus | 10x buffer | dNTP | MgSO4 | Primer(661-RBS-KaiA-fwd) | Primer(kaiA-662-rev-ver2) | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 15.5 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 57°C | 68°C | -- |
| 2min | 10s | 30s | 28s | 30cycles |
| KaiB(plasmid) | KOD plus | 10x buffer | dNTP | MgSO4 | Primer(662-RBS-KaiB-fwd-ver2) | Primer(kaiB-663-rev-ver2) | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 15.5 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 57°C | 68°C | -- |
| 2min | 10s | 30s | 28s | 30cycles |
| DT(plasmid) | KOD plus | 10x buffer | dNTP | MgSO4 | Primer(664-DT-fwd-ver2) | Primer(DT-suffix-660-rev-ver2) | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 16.4 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 57°C | 68°C | -- |
| 2min | 10s | 30s | 28s | 30cycles |
| P-KaiB(PCR prpduct) | KOD plus | 10x buffer | dNTP | MgSO4 | Primer(prefix-PkaiBC-fwd-ver2) | Primer(PkaiBC-suffix--662-rev-ver2) | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.7 | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 15.8 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 57°C | 68°C | -- |
| 2min | 10s | 30s | 28s | 30cycles |
| Plac(1)(plasmid) | KOD plus | 10x buffer | dNTP | MgSO4 | Primer(GG0-pSBIC3-fwd-ver2) | Primer(Plac-GG1-rev-ver2) | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.13 | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 16.37 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 57°C | 68°C | -- |
| 2min | 10s | 30s | 60s | 30cycles |
| KaiC(plasmid) | KOD plus | 10x buffer | dNTP | MgSO4 | Primer(GG3-RBS-KaiC-fwd-ver2) | Primer(KaiC-GG4-rev-ver2) | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 15.5 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 57°C | 68°C | -- |
| 2min | 10s | 30s | 60s | 30cycles |
 "
"