Template:Kyoto/Notebook/Sep 11
From 2013.igem.org
(Difference between revisions)
(→PCR) |
(→Restriction Enzyme Digestion) |
||
| (69 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
==Sep 11== | ==Sep 11== | ||
| + | ===Electrophoresis=== | ||
| + | |||
| + | <div class="experiment"> | ||
| + | <span class="author"></span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||1kb ladder | ||
| + | |- | ||
| + | |2||Pbad/araC+RBS-luxI-DT-1 | ||
| + | |- | ||
| + | |3||Pbad/araC+RBS-luxI-DT-1 | ||
| + | |- | ||
| + | |4||Plac+RBS-lysis-DT | ||
| + | |- | ||
| + | |5||Pcon+RBS-luxI-DT | ||
| + | |- | ||
| + | |6||1kb ladder | ||
| + | |} | ||
| + | [[File:igku_9111.jpg]]<br> | ||
| + | </div> | ||
| + | |||
===PCR=== | ===PCR=== | ||
<div class="experiment"> | <div class="experiment"> | ||
| Line 6: | Line 28: | ||
!pKaiBC(200p)||10*buffer KOD plus||dNTPs||MgSO4||primer mix(F&R)||KOD plus||MilliQ||total | !pKaiBC(200p)||10*buffer KOD plus||dNTPs||MgSO4||primer mix(F&R)||KOD plus||MilliQ||total | ||
|- | |- | ||
| - | |0.5||2.5||2.5||1.5||1.5||0.5||16 | + | |0.5||2.5||2.5||1.5||1.5||0.5||16||25 |
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 20: | Line 42: | ||
!pKaiBC(400p)||10*buffer KOD plus||dNTPs||MgSO4||primer mix(F&R)||KOD plus||MilliQ||total | !pKaiBC(400p)||10*buffer KOD plus||dNTPs||MgSO4||primer mix(F&R)||KOD plus||MilliQ||total | ||
|- | |- | ||
| - | |1||2.5||2.5||1.5||1.5||0.5||15.5 | + | |1||2.5||2.5||1.5||1.5||0.5||15.5||25 |
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 34: | Line 56: | ||
!pKaiBC(800p)||10*buffer KOD plus||dNTPs||MgSO4||primer mix(F&R)||KOD plus||MilliQ||total | !pKaiBC(800p)||10*buffer KOD plus||dNTPs||MgSO4||primer mix(F&R)||KOD plus||MilliQ||total | ||
|- | |- | ||
| - | |2||2.5||2.5||1.5||1.5||0.5||14.5 | + | |2||2.5||2.5||1.5||1.5||0.5||14.5||25 |
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 49: | Line 71: | ||
!pKaiBC(1ng)||10*buffer KOD plus||dNTPs||MgSO4||primer mix(F&R)||KOD plus||MilliQ||total | !pKaiBC(1ng)||10*buffer KOD plus||dNTPs||MgSO4||primer mix(F&R)||KOD plus||MilliQ||total | ||
|- | |- | ||
| - | |2.5||2.5||2.5||1.5||1.5||0.5||14 | + | |2.5||2.5||2.5||1.5||1.5||0.5||14||25 |
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 59: | Line 81: | ||
|} | |} | ||
</div> | </div> | ||
| + | ===Electrophoresis=== | ||
| + | |||
| + | <!-- こっから --> | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Tatsui</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||100bp ladder | ||
| + | |- | ||
| + | |2||pKaiBC 800pg 60°C | ||
| + | |- | ||
| + | |3||pKaiBC 1ng 60°C | ||
| + | |- | ||
| + | |4||pKaiBC 800pg 61°C | ||
| + | |- | ||
| + | |5||pKaiBC 1ng 61°C | ||
| + | |- | ||
| + | |6||pKaiBC 800pg 62°C | ||
| + | |- | ||
| + | |7||pKaiBC 1ng 62°C | ||
| + | |- | ||
| + | |8||100bp ladder | ||
| + | |} | ||
| + | [[File:igku_9112.jpg]]<br> | ||
| + | </div> | ||
| + | <!-- ここまでをコピーしてね --> | ||
| + | |||
===colony PCR=== | ===colony PCR=== | ||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">Tatsui</span> |
{| class="wikitable" | {| class="wikitable" | ||
!Sample||base pair | !Sample||base pair | ||
|- | |- | ||
| - | | | + | |9/9Pcon-lacZα(p3B4k5)||712bp |
| + | |- | ||
| + | |9/9Pcon-spinach-DT(p3B4k5)||605bp | ||
|} | |} | ||
| + | |||
| + | </div> | ||
| + | |||
| + | <div class="experiment"> | ||
| + | <span class="author">Tatsui</span> | ||
{| class="wikitable" | {| class="wikitable" | ||
!PreDenature||Denature||Annealing||Extension||cycle | !PreDenature||Denature||Annealing||Extension||cycle | ||
| Line 72: | Line 129: | ||
| 94°C|| 94°C|| 55°C|| 68°C||-- | | 94°C|| 94°C|| 55°C|| 68°C||-- | ||
|- | |- | ||
| - | |5min||30s ||30s ||42s || | + | |5min||30s ||30s ||42s ||30 |
|} | |} | ||
</div> | </div> | ||
| + | |||
| + | |||
<div> | <div> | ||
{| class="wikitable" | {| class="wikitable" | ||
!Sample||base pair | !Sample||base pair | ||
|- | |- | ||
| - | | | + | |Pbad\araC-RBS-luxI-DT|| |
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 86: | Line 145: | ||
| 94°C|| 94°C|| 55°C|| 68°C||-- | | 94°C|| 94°C|| 55°C|| 68°C||-- | ||
|- | |- | ||
| - | |5min||30s ||30s || | + | |5min||30s ||30s ||2min ||30 |
|} | |} | ||
</div> | </div> | ||
| - | <div> | + | |
| + | ===Electrophoresis=== | ||
| + | |||
| + | <!-- こっから --> | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Tatsui</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||100bp ladder | ||
| + | |- | ||
| + | |2||Pcon-lacZα(pSB4K5)3 | ||
| + | |- | ||
| + | |3||Pcon-lacZα(pSB4K5)4 | ||
| + | |- | ||
| + | |4||Pcon-lacZα(pSB4K5)5 | ||
| + | |- | ||
| + | |5||Pcon-lacZα(pSB4K5)6 | ||
| + | |- | ||
| + | |6||Pcon-spinach-DT(pSB4K5)3 | ||
| + | |- | ||
| + | |7||Pcon-spinach-DT(pSB4K5)4 | ||
| + | |- | ||
| + | |8||Pcon-spinach-DT(pSB4K5)5 | ||
| + | |- | ||
| + | |9||Pcon-spinach-DT(pSB4K5)6 | ||
| + | |- | ||
| + | |10||100bp ladder | ||
| + | |} | ||
| + | [[File:igku_9113.jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||1kbp ladder | ||
| + | |- | ||
| + | |2||Pbad/araC-RBS-luxI-DT3 | ||
| + | |- | ||
| + | |3||Pbad/araC-RBS-luxI-DT4 | ||
| + | |- | ||
| + | |4||Pbad/araC-RBS-luxI-DT5 | ||
| + | |- | ||
| + | |5||Pbad/araC-RBS-luxI-DT6 | ||
| + | |- | ||
| + | |6||1kbp ladder | ||
| + | |} | ||
| + | [[File:igku_9114.jpg]]<br> | ||
| + | </div> | ||
| + | |||
| + | ===colony PCR=== | ||
| + | <div class="experiment"> | ||
{| class="wikitable" | {| class="wikitable" | ||
!Sample||base pair | !Sample||base pair | ||
|- | |- | ||
| - | | | + | |9/9Pcon+RBS-luxI-DT||1079bp |
| + | |- | ||
| + | |9/10Pcon+sptamer12_1R-DT||584bp | ||
| + | |- | ||
| + | |9/10DT+Pcon-pT181 attenuator 1||781bp | ||
| + | |- | ||
| + | |9/10Fusion1 attenuator(p3B1C3)-1||638bp | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 100: | Line 214: | ||
| 94°C|| 94°C|| 55°C|| 68°C||-- | | 94°C|| 94°C|| 55°C|| 68°C||-- | ||
|- | |- | ||
| - | |5min|| | + | |50sec ||30sec ||30sec ||1min ||30 |
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Gel Extraction=== | ||
| + | <!-- こっから --> | ||
| + | <div class="experiment"> | ||
| + | <span class="author"></span> | ||
| + | [[File:igku_9115.jpg]]<br> | ||
| + | [[File:igku_9116.jpg]]<br> | ||
| + | [[File:igku_9117.jpg]]<br> | ||
| + | [[File:igku_9118.jpg]]<br> | ||
| + | </div> | ||
| + | <!-- ここまでをコピーしてね --> | ||
| + | |||
| + | ===Ligation=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Okazaki</span> | ||
| + | {| class="wikitable" | ||
| + | !state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | ||
| + | |- | ||
| + | |experiment||9/8pSB4K5(EcoRI&SpeI)21.8ng/µL||2.3 µL||9/8Pcon-Spinach-DT(EcoRI&SpeI)10.5ng/µL||1.7 µL||2.0 µL | ||
| + | |- | ||
| + | |experment||9/8pSB4K5(EcoRI&SpeI)21.8ng/µL||2.3 µL||9/8RBS-lacZα-DT(EcoRI&SpeI)4.4ng/µL|| 6.7µL||3.5 µL | ||
| + | |- | ||
| + | |experiment||9/8pSB4K5(EcoRI&SpeI)21.8ng/µL||2.3 µL||9/8Pcon-RBS-lacZα-DT(EcoRI&SpeI)5.6ng/µL||1.9 µL||2.1 µL | ||
| + | |- | ||
| + | |experiment||9/6Plux 25ng/µL||2 µL||9/8 RBS-lysis1-DT(XbaI&PstI)||6.6 µL||3.5 µL | ||
| + | |- | ||
| + | |experiment||9/6Plux 25ng/µL||2 µL||9/8 RBS-lysis3-DT(XbaI&PstI)||4.7 µL||3.4 µL | ||
| + | |- | ||
| + | |experiment||9/10Pcon-pT181 attenuator(SpeI&PstI)|| 3.4µL||9/8RBS-lacZα-DT(EcoRI&SpeI)||4.5 µL||3.5 µL | ||
| + | |- | ||
| + | |experiment||9/6Pcon-RBS laxR-DT(EcoRI&XbaI) 26.7ng/µL||1.9 µL||Plax-RBS-GFP-DT||5.5 µL||3.5 µL | ||
| + | |- | ||
| + | |experiment||9/9 Pcon-pT181 attenuator(SpeI&PstI)||3.4 µL||9/10 aptamer12_1R-DT(XbaI&PstI)||1.7 µL||2.6 µL | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Transformation=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto Tatsui</span> | ||
| + | {| class="wikitable" | ||
| + | !Name||Sample||Competent Cells||Total||Plate | ||
| + | |- | ||
| + | |9/9 Fusion1 antisense(pSB2C3)||2µL||20µL||22µL||CP | ||
| + | |- | ||
| + | |9/9 Fusion6 antisense(pSB2C3)||2µL||20µL||22µL||CP | ||
| + | |- | ||
| + | |9/9 aptamer 12_P(pSB2C3)||2µL||20µL||22µL||CP | ||
| + | |- | ||
| + | |9/9 aptamer 12_1µ(pSB2C3)||2µL||20µL||22µL||CP | ||
| + | |- | ||
| + | |9/9 Fusion2 attenuator(pSB2C3) ||2µL||20µL||22µL||CP | ||
| + | |- | ||
| + | |9/11 Pcon-spinach-DT(pSB4K5)||2µL||20µL||22µL||K | ||
| + | |- | ||
| + | |9/11 RBS-lacZα-DT(pSB4K5)||2µL||20µL||22µL||K | ||
| + | |- | ||
| + | |9/11 Pcon-RBS-lacZα-DT(pSB4K5)||2µL||20µL||22µL||K | ||
| + | |- | ||
| + | |Plux+RBS-lysis1-DT||2µL||20µL||22µL||CP | ||
| + | |- | ||
| + | |Plux+RBS-lysis3-DT||2µL||20µL||22µL||CP | ||
| + | |- | ||
| + | |Pcon-pT181attenuator+RBS-lacZα-DT||2µL||20µL||22µL||Amp | ||
| + | |- | ||
| + | |Pcon-iuxR+Plux-GFP||2µL||20µL||22µL||Amp | ||
| + | |- | ||
| + | |Pcon-pT181attenuator+apt12_1R-DT||2µL||20µL||22µL||Amp | ||
| + | |- | ||
| + | |Plac(BBa_R0011)||2µL||20µL||22µL||Amp|| | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Electrophoresis=== | ||
| + | |||
| + | <!-- こっから --> | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Tatsui</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||100bp ladder | ||
| + | |- | ||
| + | |2||Pcon+RBS luxI-DT2 | ||
| + | |- | ||
| + | |3||Pcon+RBS luxI-DT3 | ||
| + | |- | ||
| + | |4||Pcon+RBS luxI-DT4 | ||
| + | |- | ||
| + | |5||Pcon+RBS luxI-DT5 | ||
| + | |- | ||
| + | |6||Pcon+RBS luxI-DT6 | ||
| + | |- | ||
| + | |7||Pcon+apF12_1R-DT1 | ||
| + | |- | ||
| + | |8||Pcon+apF12_1R-DT2 | ||
| + | |- | ||
| + | |9||Pcon+apF12_1R-DT3 | ||
| + | |- | ||
| + | |10||Pcon+apF12_1R-DT4 | ||
| + | |- | ||
| + | |11||DT+Pcon-pT181attenuator1 | ||
| + | |- | ||
| + | |12||F1attenuator(pSB1C3) | ||
| + | |- | ||
| + | |13||100bp ladder | ||
| + | |} | ||
| + | [[File:igku_9119.jpg]]<br> | ||
| + | </div> | ||
| + | |||
| + | ===Restriction Enzyme Digestion=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto</span> | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||EcoRI||XbaI||SpeI||PstI||buffer||BSA||MilliQ||Total | ||
| + | |- | ||
| + | |pSB4K5(EcoRI+SpeI)||9.4µL|| 1µL|| 0µL|| 1µL|| 0µL|| 3µL|| 3µL|| 12.6µL||30 µL | ||
| + | |- | ||
| + | |pSB4K5(EcoRI+SpeI) negative||0.5µL|| 0µL|| 0µL|| 0µL|| 0µL|| 1µL|| 1µL|| 7.5µL|| 10µL | ||
| + | |- | ||
| + | |RBS-lacZα-DT(EcoRI+SpeI)|| 10µL|| 1µL|| 0µL|| 1µL|| 0µL|| 3µL|| 3µL|| 12µL|| 30µL | ||
| + | |- | ||
| + | |RBS-lacZα-DT(EcoRI+SpeI) negative|| 0.5µL|| 0µL|| 0µL|| 0µL|| 0µL|| 1µL|| 1µL|| 7.5µL|| 10µL | ||
| + | |- | ||
| + | |Ptet-pT181 antisense(SteI+PstI)|| 10.8µL|| 0µL|| 0µL|| 1µL|| 1µL|| 3µL|| 3µL|| 12µL|| 30µL | ||
| + | |- | ||
| + | |Ptet-pT181 antisense(SteI+PstI) negative|| 0.5µL|| 0µL|| 0µL|| 0µL|| 0µL|| 1µL|| 1µL|| 7.5µL|| 10µL | ||
| + | |- | ||
| + | |Pcon-RBS-lacZα-DT(EcoRI+SpeI)|| 7.1µL|| 1µL|| 0µL|| 1µL|| 0µL|| 3µL|| 3µL|| 14.9µL|| 30µL | ||
| + | |- | ||
| + | |Pcon-RBS-lacZα-DT(EcoRI+SpeI) negative|| 0.4µL|| 0µL|| 0µL|| 0µL|| 0µL|| 1µL|| 1µL|| 7.6µL|| 10µL | ||
| + | |- | ||
| + | |RBS-lysis2-DT(XbaI+PstI)|| 22µL|| 0µL|| 1µL|| 0µL|| 1µL|| 3µL|| 3µL|| 0µL|| 30µL | ||
| + | |- | ||
| + | |RBS-lysis2-DT(XbaI+PstI) negative|| 1.4µL|| 0µL|| 0µL|| 0µL|| 0µL|| 1µL|| 1µL|| 6.6µL|| 10µL | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Liquid Culture=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||medium | ||
| + | |- | ||
| + | |8/16 Master4 Plax|| | ||
| + | |- | ||
| + | |8/25 Pλ-laxI-2 || | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===PCR=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Hirano</span> | ||
| + | {| class="wikitable" | ||
| + | !8/29 Pλ-RBS-luxI-DT(76.5ng/µL)||10x buffer for KOD plus-ver.2||KOD-plus||dNTP||MgSO4||F primer(RBS-luxI-DT-cloning-fwd)||R primer(RBS-luxI-DT-cloning-rev)||MilliQ||total | ||
| + | |- | ||
| + | |0.3||2.5||0.5||2.5||1.5||0.75||0.75||16.2||25 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | | 94°C|| 94°C|| 65°C|| 68°C||-- | ||
| + | |- | ||
| + | |5min||30sec||30sec||1min||30 | ||
| + | |} | ||
| + | |||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | !8/31 Pbad/araC-RBS-RFP(1)(67.6ng/µL)||10x buffer for KOD plus-ver.2||KOD-plus||dNTP||MgSO4||F primer(Pbad/araC(pSBlc3)-cloning-fwd)||R primer(Pbad/araC(pSBlc3)-cloning-rev)||MilliQ||total | ||
| + | |- | ||
| + | |0.3||2.5||0.5||2.5||1.5||0.75||0.75||16.2||25 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | | 94°C|| 94°C|| 54°C|| 68°C||-- | ||
| + | |- | ||
| + | |5min||30sec||30sec||3min 20sec||30 | ||
| + | |} | ||
| + | |||
| + | ===Liquid Culture=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Hirano</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||medium | ||
| + | |- | ||
| + | |9/4 RBS-lysis1+DT 3||plusgrow(+CP) 3ml 37°C | ||
| + | |- | ||
| + | |9/4 RBS-lysis2+DT 1||plusgrow(+CP) 3ml 37°C | ||
| + | |- | ||
| + | |9/4 RBS-lysis3+DT 3||plusgrow(+CP) 3ml 37°C | ||
|} | |} | ||
</div> | </div> | ||
Latest revision as of 15:00, 27 September 2013
Contents |
Sep 11
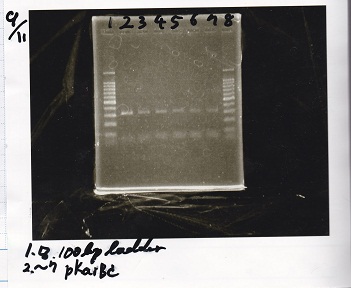
Electrophoresis
| Lane | Sample |
|---|---|
| 1 | 1kb ladder |
| 2 | Pbad/araC+RBS-luxI-DT-1 |
| 3 | Pbad/araC+RBS-luxI-DT-1 |
| 4 | Plac+RBS-lysis-DT |
| 5 | Pcon+RBS-luxI-DT |
| 6 | 1kb ladder |
PCR
| pKaiBC(200p) | 10*buffer KOD plus | dNTPs | MgSO4 | primer mix(F&R) | KOD plus | MilliQ | total |
|---|---|---|---|---|---|---|---|
| 0.5 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 16 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 60°C | 68°C | 30 |
| 2min | 10sec | 30sec | 30sec |
| pKaiBC(400p) | 10*buffer KOD plus | dNTPs | MgSO4 | primer mix(F&R) | KOD plus | MilliQ | total |
|---|---|---|---|---|---|---|---|
| 1 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 15.5 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 60°C | 68°C | 30 |
| 2min | 10sec | 30sec | 30sec |
| pKaiBC(800p) | 10*buffer KOD plus | dNTPs | MgSO4 | primer mix(F&R) | KOD plus | MilliQ | total |
|---|---|---|---|---|---|---|---|
| 2 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 14.5 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 60°C | 68°C | 30 |
| 2min | 10sec | 30sec | 30sec |
| pKaiBC(1ng) | 10*buffer KOD plus | dNTPs | MgSO4 | primer mix(F&R) | KOD plus | MilliQ | total |
|---|---|---|---|---|---|---|---|
| 2.5 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 14 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 60°C | 68°C | 30 |
| 2min | 10sec | 30sec | 30sec |
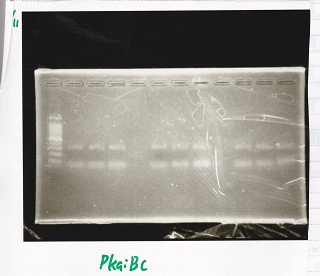
Electrophoresis
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | pKaiBC 800pg 60°C |
| 3 | pKaiBC 1ng 60°C |
| 4 | pKaiBC 800pg 61°C |
| 5 | pKaiBC 1ng 61°C |
| 6 | pKaiBC 800pg 62°C |
| 7 | pKaiBC 1ng 62°C |
| 8 | 100bp ladder |
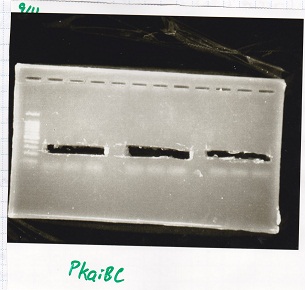
colony PCR
| Sample | base pair |
|---|---|
| 9/9Pcon-lacZα(p3B4k5) | 712bp |
| 9/9Pcon-spinach-DT(p3B4k5) | 605bp |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 42s | 30 |
| Sample | base pair |
|---|---|
| Pbad\araC-RBS-luxI-DT |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 2min | 30 |
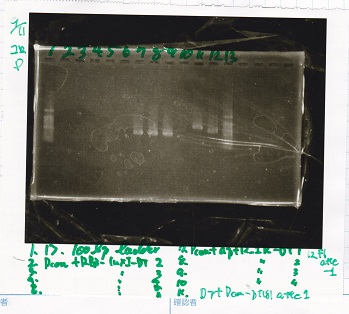
Electrophoresis
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | Pcon-lacZα(pSB4K5)3 |
| 3 | Pcon-lacZα(pSB4K5)4 |
| 4 | Pcon-lacZα(pSB4K5)5 |
| 5 | Pcon-lacZα(pSB4K5)6 |
| 6 | Pcon-spinach-DT(pSB4K5)3 |
| 7 | Pcon-spinach-DT(pSB4K5)4 |
| 8 | Pcon-spinach-DT(pSB4K5)5 |
| 9 | Pcon-spinach-DT(pSB4K5)6 |
| 10 | 100bp ladder |
| Lane | Sample |
|---|---|
| 1 | 1kbp ladder |
| 2 | Pbad/araC-RBS-luxI-DT3 |
| 3 | Pbad/araC-RBS-luxI-DT4 |
| 4 | Pbad/araC-RBS-luxI-DT5 |
| 5 | Pbad/araC-RBS-luxI-DT6 |
| 6 | 1kbp ladder |
colony PCR
| Sample | base pair |
|---|---|
| 9/9Pcon+RBS-luxI-DT | 1079bp |
| 9/10Pcon+sptamer12_1R-DT | 584bp |
| 9/10DT+Pcon-pT181 attenuator 1 | 781bp |
| 9/10Fusion1 attenuator(p3B1C3)-1 | 638bp |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 50sec | 30sec | 30sec | 1min | 30 |
Gel Extraction
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | 9/8pSB4K5(EcoRI&SpeI)21.8ng/µL | 2.3 µL | 9/8Pcon-Spinach-DT(EcoRI&SpeI)10.5ng/µL | 1.7 µL | 2.0 µL |
| experment | 9/8pSB4K5(EcoRI&SpeI)21.8ng/µL | 2.3 µL | 9/8RBS-lacZα-DT(EcoRI&SpeI)4.4ng/µL | 6.7µL | 3.5 µL |
| experiment | 9/8pSB4K5(EcoRI&SpeI)21.8ng/µL | 2.3 µL | 9/8Pcon-RBS-lacZα-DT(EcoRI&SpeI)5.6ng/µL | 1.9 µL | 2.1 µL |
| experiment | 9/6Plux 25ng/µL | 2 µL | 9/8 RBS-lysis1-DT(XbaI&PstI) | 6.6 µL | 3.5 µL |
| experiment | 9/6Plux 25ng/µL | 2 µL | 9/8 RBS-lysis3-DT(XbaI&PstI) | 4.7 µL | 3.4 µL |
| experiment | 9/10Pcon-pT181 attenuator(SpeI&PstI) | 3.4µL | 9/8RBS-lacZα-DT(EcoRI&SpeI) | 4.5 µL | 3.5 µL |
| experiment | 9/6Pcon-RBS laxR-DT(EcoRI&XbaI) 26.7ng/µL | 1.9 µL | Plax-RBS-GFP-DT | 5.5 µL | 3.5 µL |
| experiment | 9/9 Pcon-pT181 attenuator(SpeI&PstI) | 3.4 µL | 9/10 aptamer12_1R-DT(XbaI&PstI) | 1.7 µL | 2.6 µL |
Transformation
| Name | Sample | Competent Cells | Total | Plate | |
|---|---|---|---|---|---|
| 9/9 Fusion1 antisense(pSB2C3) | 2µL | 20µL | 22µL | CP | |
| 9/9 Fusion6 antisense(pSB2C3) | 2µL | 20µL | 22µL | CP | |
| 9/9 aptamer 12_P(pSB2C3) | 2µL | 20µL | 22µL | CP | |
| 9/9 aptamer 12_1µ(pSB2C3) | 2µL | 20µL | 22µL | CP | |
| 9/9 Fusion2 attenuator(pSB2C3) | 2µL | 20µL | 22µL | CP | |
| 9/11 Pcon-spinach-DT(pSB4K5) | 2µL | 20µL | 22µL | K | |
| 9/11 RBS-lacZα-DT(pSB4K5) | 2µL | 20µL | 22µL | K | |
| 9/11 Pcon-RBS-lacZα-DT(pSB4K5) | 2µL | 20µL | 22µL | K | |
| Plux+RBS-lysis1-DT | 2µL | 20µL | 22µL | CP | |
| Plux+RBS-lysis3-DT | 2µL | 20µL | 22µL | CP | |
| Pcon-pT181attenuator+RBS-lacZα-DT | 2µL | 20µL | 22µL | Amp | |
| Pcon-iuxR+Plux-GFP | 2µL | 20µL | 22µL | Amp | |
| Pcon-pT181attenuator+apt12_1R-DT | 2µL | 20µL | 22µL | Amp | |
| Plac(BBa_R0011) | 2µL | 20µL | 22µL | Amp |
Electrophoresis
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | Pcon+RBS luxI-DT2 |
| 3 | Pcon+RBS luxI-DT3 |
| 4 | Pcon+RBS luxI-DT4 |
| 5 | Pcon+RBS luxI-DT5 |
| 6 | Pcon+RBS luxI-DT6 |
| 7 | Pcon+apF12_1R-DT1 |
| 8 | Pcon+apF12_1R-DT2 |
| 9 | Pcon+apF12_1R-DT3 |
| 10 | Pcon+apF12_1R-DT4 |
| 11 | DT+Pcon-pT181attenuator1 |
| 12 | F1attenuator(pSB1C3) |
| 13 | 100bp ladder |
Restriction Enzyme Digestion
| DNA | EcoRI | XbaI | SpeI | PstI | buffer | BSA | MilliQ | Total | |
|---|---|---|---|---|---|---|---|---|---|
| pSB4K5(EcoRI+SpeI) | 9.4µL | 1µL | 0µL | 1µL | 0µL | 3µL | 3µL | 12.6µL | 30 µL |
| pSB4K5(EcoRI+SpeI) negative | 0.5µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL |
| RBS-lacZα-DT(EcoRI+SpeI) | 10µL | 1µL | 0µL | 1µL | 0µL | 3µL | 3µL | 12µL | 30µL |
| RBS-lacZα-DT(EcoRI+SpeI) negative | 0.5µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL |
| Ptet-pT181 antisense(SteI+PstI) | 10.8µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 12µL | 30µL |
| Ptet-pT181 antisense(SteI+PstI) negative | 0.5µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL |
| Pcon-RBS-lacZα-DT(EcoRI+SpeI) | 7.1µL | 1µL | 0µL | 1µL | 0µL | 3µL | 3µL | 14.9µL | 30µL |
| Pcon-RBS-lacZα-DT(EcoRI+SpeI) negative | 0.4µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.6µL | 10µL |
| RBS-lysis2-DT(XbaI+PstI) | 22µL | 0µL | 1µL | 0µL | 1µL | 3µL | 3µL | 0µL | 30µL |
| RBS-lysis2-DT(XbaI+PstI) negative | 1.4µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 6.6µL | 10µL |
Liquid Culture
| Sample | medium |
|---|---|
| 8/16 Master4 Plax | |
| 8/25 Pλ-laxI-2 |
PCR
| 8/29 Pλ-RBS-luxI-DT(76.5ng/µL) | 10x buffer for KOD plus-ver.2 | KOD-plus | dNTP | MgSO4 | F primer(RBS-luxI-DT-cloning-fwd) | R primer(RBS-luxI-DT-cloning-rev) | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.3 | 2.5 | 0.5 | 2.5 | 1.5 | 0.75 | 0.75 | 16.2 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 65°C | 68°C | -- |
| 5min | 30sec | 30sec | 1min | 30 |
| 8/31 Pbad/araC-RBS-RFP(1)(67.6ng/µL) | 10x buffer for KOD plus-ver.2 | KOD-plus | dNTP | MgSO4 | F primer(Pbad/araC(pSBlc3)-cloning-fwd) | R primer(Pbad/araC(pSBlc3)-cloning-rev) | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.3 | 2.5 | 0.5 | 2.5 | 1.5 | 0.75 | 0.75 | 16.2 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 54°C | 68°C | -- |
| 5min | 30sec | 30sec | 3min 20sec | 30 |
Liquid Culture
| Sample | medium |
|---|---|
| 9/4 RBS-lysis1+DT 3 | plusgrow(+CP) 3ml 37°C |
| 9/4 RBS-lysis2+DT 1 | plusgrow(+CP) 3ml 37°C |
| 9/4 RBS-lysis3+DT 3 | plusgrow(+CP) 3ml 37°C |
 "
"