Team:UGA-Georgia/Plan
From 2013.igem.org
(→General Purpose and Goal) |
Hamptonite (Talk | contribs) (→Objectives) |
||
| (5 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
=General Purpose and Goal = | =General Purpose and Goal = | ||
| - | The first purpose for this study is to determine if a gene for geraniol synthase can be properly expressed in the archaea ''Methanococcus maripaludis'' via a plasmid shuttle vector. Successful transformation will result in ''Methanococcus maripaludis'' producing geraniol from geranyl pyrophosphate, an intermediate in a | + | The first purpose for this study is to determine if a gene for geraniol synthase can be properly expressed in the archaea ''Methanococcus maripaludis'' via a plasmid shuttle vector. Successful transformation will result in ''Methanococcus maripaludis'' producing geraniol from geranyl pyrophosphate, an intermediate in a lipid biosynthesis pathway. |
The second plan is to develop novel gene expression and quantification tools for ''Methanogens''. To achieve this we will design novel vectors with the potential to express red fluorescence. This vector will have the additional capability of allowing users to directly fuse their protein of interest with the red fluorescent protein. | The second plan is to develop novel gene expression and quantification tools for ''Methanogens''. To achieve this we will design novel vectors with the potential to express red fluorescence. This vector will have the additional capability of allowing users to directly fuse their protein of interest with the red fluorescent protein. | ||
| Line 9: | Line 9: | ||
= Objectives = | = Objectives = | ||
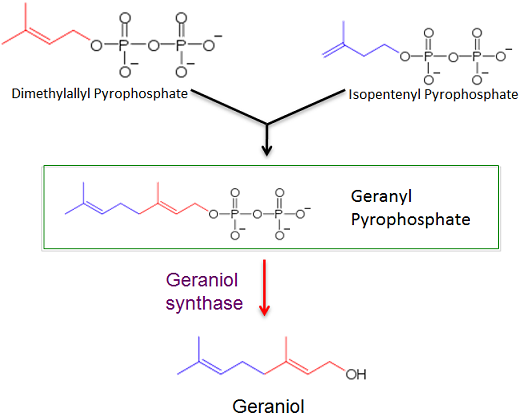
| - | 1. | + | 1. Our first objective was to create a plasmid shuttle vector that would effectively express a gene for geraniol synthase in ''Methanococcus maripaludis'' and produce geraniol. The geraniol synthase gene catalyzes the transition between geranyl pyrophosphate, a natural intermediate in a lipid biosynthesis pathway in Methanococcus, into geraniol. Geraniol is a high-value chemical with many uses including inhibiting cancer growth, biofuel and fragrances. We could evaluate and quantify geraniol production in Methanococcus using Gas Chromatography and Mass Spectroscopy techniques. |
[[Image:UGA Biosynth Path.png|center]] | [[Image:UGA Biosynth Path.png|center]] | ||
| Line 17: | Line 17: | ||
[[Image:Biobrick v2v4.png|center]] | [[Image:Biobrick v2v4.png|center]] | ||
| - | 3. To increase the awareness of synthetic biology | + | 3. To increase the awareness of synthetic biology to the community. |
= Plan = | = Plan = | ||
Genetic transformation will be accomplished through the use of a plasmid shuttle vector, pAW50. The DNA sequences encoding for geraniol synthase and mcherry will be added to the pAW50 vector and transformed into ''E. coli''. The sequences were slightly altered for better expression in ''M. maripaludis'' by way of codon optimization. This technique involves using the most frequent base pair triplets which appear most often for a certain codon in the ''M. maripaludis'' genome. After the genes are inserted into the vector they are transformed into cells of the ''E. coli'' strain XL-1 Blue to replicate the plasmid. The ''E. coli'' cells will then be lysed to extract the plasmids for transformation into ''M. maripaludis''. The plasmid will also contain a gene encoding for a resistance to the antibiotic puromycin. This will serve as a selection marker in order to confirm successful transformation of the plasmid. Cells which have not taken up a plasmid will die when grown on media that contains puromycin. Fluorescent studies will be accomplished using the plate reader available in Dr. Yan's Lab. | Genetic transformation will be accomplished through the use of a plasmid shuttle vector, pAW50. The DNA sequences encoding for geraniol synthase and mcherry will be added to the pAW50 vector and transformed into ''E. coli''. The sequences were slightly altered for better expression in ''M. maripaludis'' by way of codon optimization. This technique involves using the most frequent base pair triplets which appear most often for a certain codon in the ''M. maripaludis'' genome. After the genes are inserted into the vector they are transformed into cells of the ''E. coli'' strain XL-1 Blue to replicate the plasmid. The ''E. coli'' cells will then be lysed to extract the plasmids for transformation into ''M. maripaludis''. The plasmid will also contain a gene encoding for a resistance to the antibiotic puromycin. This will serve as a selection marker in order to confirm successful transformation of the plasmid. Cells which have not taken up a plasmid will die when grown on media that contains puromycin. Fluorescent studies will be accomplished using the plate reader available in Dr. Yan's Lab. | ||
Latest revision as of 17:44, 27 September 2013
General Purpose and Goal
The first purpose for this study is to determine if a gene for geraniol synthase can be properly expressed in the archaea Methanococcus maripaludis via a plasmid shuttle vector. Successful transformation will result in Methanococcus maripaludis producing geraniol from geranyl pyrophosphate, an intermediate in a lipid biosynthesis pathway.
The second plan is to develop novel gene expression and quantification tools for Methanogens. To achieve this we will design novel vectors with the potential to express red fluorescence. This vector will have the additional capability of allowing users to directly fuse their protein of interest with the red fluorescent protein.
Objectives
1. Our first objective was to create a plasmid shuttle vector that would effectively express a gene for geraniol synthase in Methanococcus maripaludis and produce geraniol. The geraniol synthase gene catalyzes the transition between geranyl pyrophosphate, a natural intermediate in a lipid biosynthesis pathway in Methanococcus, into geraniol. Geraniol is a high-value chemical with many uses including inhibiting cancer growth, biofuel and fragrances. We could evaluate and quantify geraniol production in Methanococcus using Gas Chromatography and Mass Spectroscopy techniques.
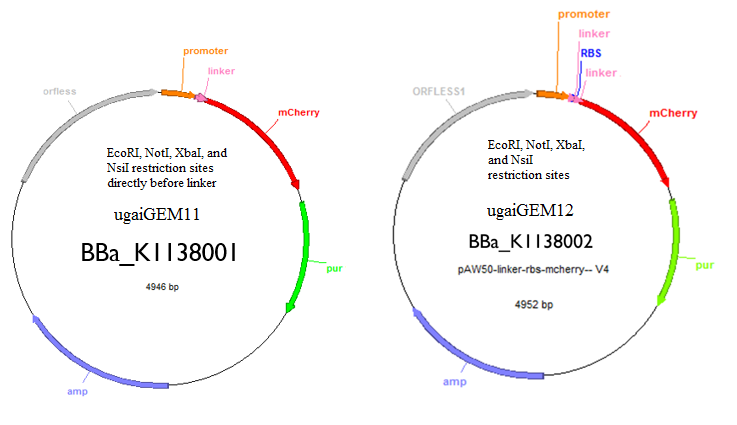
2. Our second objective was creating a tool that will allow future researchers and iGEM teams to quantify and regulate gene expression in Archaea.
3. To increase the awareness of synthetic biology to the community.
Plan
Genetic transformation will be accomplished through the use of a plasmid shuttle vector, pAW50. The DNA sequences encoding for geraniol synthase and mcherry will be added to the pAW50 vector and transformed into E. coli. The sequences were slightly altered for better expression in M. maripaludis by way of codon optimization. This technique involves using the most frequent base pair triplets which appear most often for a certain codon in the M. maripaludis genome. After the genes are inserted into the vector they are transformed into cells of the E. coli strain XL-1 Blue to replicate the plasmid. The E. coli cells will then be lysed to extract the plasmids for transformation into M. maripaludis. The plasmid will also contain a gene encoding for a resistance to the antibiotic puromycin. This will serve as a selection marker in order to confirm successful transformation of the plasmid. Cells which have not taken up a plasmid will die when grown on media that contains puromycin. Fluorescent studies will be accomplished using the plate reader available in Dr. Yan's Lab.
 "
"