Template:Kyoto/Notebook/Sep 13
From 2013.igem.org
(Difference between revisions)
(Created page with "==Sep 13==") |
(→PCR) |
||
| (35 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
==Sep 13== | ==Sep 13== | ||
| + | ===Miniprep=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto</span> | ||
| + | {|class="wikitable" | ||
| + | !DNA||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |9/12 Fusion6 antisense||245.9||1.88||1.41 | ||
| + | |- | ||
| + | |9/12 Plux-3||115.5||1.58||1.32 | ||
| + | |- | ||
| + | |9/12 Plux-4||125.3||1.93||1.44 | ||
| + | |- | ||
| + | |9/12 Fusion3n2 attenuator-1||231.9||1.81||1.31 | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Gel Extraction=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Tatsui,Ashida</span> | ||
| + | {| class="wikitable" | ||
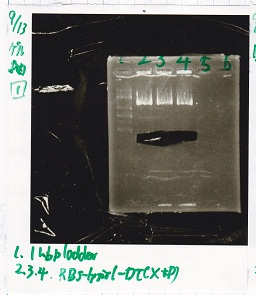
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||1kb ladder||-- | ||
| + | |- | ||
| + | |2||RBS-lysis1-DT||XbaI&PstI | ||
| + | |- | ||
| + | |3||RBS-lysis1-DT||XbaI&PstI | ||
| + | |- | ||
| + | |4||RBS-lysis1-DT||XbaI&PstI | ||
| + | |} | ||
| + | |||
| + | [[File:igku_9131.jpg]]<br> | ||
| + | [[File:igku_9132.jpg]]<br> | ||
| + | |||
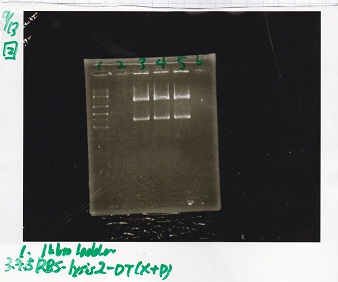
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||1kb ladder||-- | ||
| + | |- | ||
| + | |3||RBS-lysis2-DT||XbaI&PstI | ||
| + | |- | ||
| + | |4||RBS-lysis2-DT||XbaI&PstI | ||
| + | |- | ||
| + | |5||RBS-lysis2-DT||XbaI&PstI | ||
| + | |} | ||
| + | |||
| + | [[File:igku_9133.jpg]]<br> | ||
| + | [[File:igku_9134.jpg]]<br> | ||
| + | |||
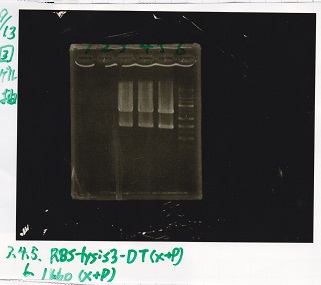
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |3||RBS-lysis3-DT||XbaI&PstI | ||
| + | |- | ||
| + | |4||RBS-lysis3-DT||XbaI&PstI | ||
| + | |- | ||
| + | |5||RBS-lysis3-DT||XbaI&PstI | ||
| + | |- | ||
| + | |6||1kb ladder||-- | ||
| + | |} | ||
| + | |||
| + | [[File:igku_9135.jpg]]<br> | ||
| + | [[File:igku_9136.jpg]]<br> | ||
| + | |||
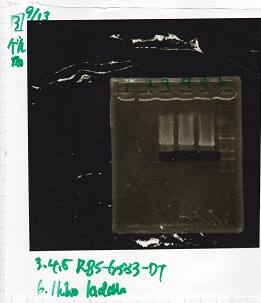
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |2||1kb ladder||-- | ||
| + | |- | ||
| + | |3||pSB1C3||EcoRI&SpeI | ||
| + | |- | ||
| + | |4||pSB1C3||EcoRI&SpeI | ||
| + | |- | ||
| + | |5||pSB1C3||EcoRI&SpeI | ||
| + | |||
| + | |} | ||
| + | [[File:igku_9137.jpg]]<br> | ||
| + | [[File:igku_9138.jpg]]<br> | ||
| + | |||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||1kb ladder||-- | ||
| + | |- | ||
| + | |2||pSB1C3||XbaI&PstI | ||
| + | |- | ||
| + | |3||pSB1C3||XbaI&PstI | ||
| + | |- | ||
| + | |4||pSB1C3||XbaI&PstI | ||
| + | |} | ||
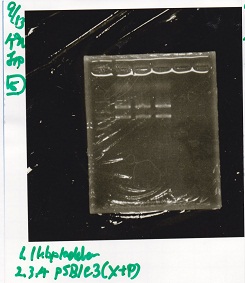
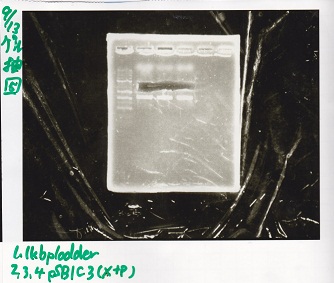
| + | [[File:igku_9139.jpg]]<br> | ||
| + | [[File:igku_91310.jpg]]<br> | ||
| + | |||
| + | ===Restriction Enzyme Digestion=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto</span> | ||
| + | {| class="wikitable" | ||
| + | ! ||9/13 Plac||EcoRI||SpeI||XbaI||PstI|| Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |1cuts(PstI)||16||0µL||0µL||0µL||1µL||3µL||3µL||7µL||30µL | ||
| + | |- | ||
| + | |NC||0.8||0µL||0µL||0µL||0µL||1µL||1µL||7.2µL||10µL | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | ! ||9/10 Pcon attenuator||EcoRI||SpeI||XbaI||PstI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |1cut(PstI)||10||0µL||0µL||0µL||1µL||3µL||3µL||13µL||30µL | ||
| + | |- | ||
| + | |NC||0.5||0µL||0µL||0µL||0µL||1µL||1µL||7.5µL||10µL | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | ! ||9/12 Plux ||EcoRI||SpeI||XbaI||PstI|| Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |1cuts(PstI)||12||0µL||0µL||0µL||1µL||3µL||3µL||11µL||30µL | ||
| + | |- | ||
| + | |NC||0.6||0µL||0µL||0µL||0µL||1µL||1µL||7.4µL||10µL | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | ! ||9/12 DT-E||EcoRI||SpeI||XbaI||PstI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |1cut(XbaI)||23.7||0µL||0µL||1µL||0µL||3µL||3µL||0µL||30.7µL | ||
| + | |- | ||
| + | |NC||1.2||0µL||0µL||0µL||0µL||1µL||1µL||6.8µL||10µL | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | ! ||9/12 Pcon-RBS-luxR-bTE ||EcoRI||SpeI||XbaI||PstI|| Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |1cuts(XbaI)||22.4||0µL||0µL||1µL||0µL||3µL||3µL||0.6µL||30µL | ||
| + | |- | ||
| + | |NC||2.2||0µL||0µL||0µL||0µL||1µL||1µL||5.8µL||10µL | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | ! ||9/12 Pcon-P||EcoRI||SpeI||XbaI||PstI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |1cut(SpeI)||21||0µL||1µL||0µL||0µL||3µL||3µL||12.0µL||30µL | ||
| + | |- | ||
| + | |NC||2.1||0µL||0µL||0µL||0µL||1µL||1µL||5.7µL||10µL | ||
| + | |} | ||
| + | |||
| + | ===M9 (EDTA)=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Kanbayashi</span> | ||
| + | 1mM EDTA</div> | ||
| + | {| class="wikitable" | ||
| + | !volume||10ml | ||
| + | |- | ||
| + | |Na2HPO4|| 300mg | ||
| + | |- | ||
| + | |KH2PO4||150mg | ||
| + | |- | ||
| + | |NaCl||25mg | ||
| + | |- | ||
| + | |NH4Cl||50mg | ||
| + | |- | ||
| + | |Fe(3)-EDTA||21mg | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | 5mM EDTA</div> | ||
| + | {| class="wikitable" | ||
| + | !volume||10ml | ||
| + | |- | ||
| + | |Na2HPO4||300mg | ||
| + | |- | ||
| + | |KH2PO4||150mg | ||
| + | |- | ||
| + | |NaCl||25mg | ||
| + | |- | ||
| + | |NH4Cl||50mg | ||
| + | |- | ||
| + | |Fe(3)-EDTA||105mg | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Gel Extraction=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||1kb ladder||-- | ||
| + | |- | ||
| + | |3||Pcon-P||SpeI | ||
| + | |- | ||
| + | |4||Pcon-P||SpeI | ||
| + | |- | ||
| + | |5||Pcon-P||SpeI | ||
| + | |} | ||
| + | |||
| + | [[File:igku_91311.jpg]]<br> | ||
| + | [[File:igku_91312.jpg]]<br> | ||
| + | |||
| + | ===Liquid Culture=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Tatsui</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||medium | ||
| + | |- | ||
| + | |9/11 aptamer12_1M(pSB1C3)-1||-- | ||
| + | |- | ||
| + | |9/11 aptamer 12_P1||-- | ||
| + | |- | ||
| + | |9/11 Fusaion1 antisense-4||-- | ||
| + | |- | ||
| + | |9/12 pSB4K5-1||-- | ||
| + | |- | ||
| + | |9/12 pSB4K5-2||-- | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Restriction Enzyme Digestion=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto</span> | ||
| + | {| class="wikitable" | ||
| + | ! ||9/13 Plac-P||EcoRI||SpeI||XbaI||PstI|| Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |1cuts(SpeI)||23.5||0µL||0.5µL||0µL||0µL||3µL||3µL||0µL||30µL | ||
| + | |- | ||
| + | |NC||8||0µL||0µL||0µL||0µL||1µL||1µL||0µL||10µL | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | ! ||9/13 Pcon-pT181 attenuator-P||EcoRI||SpeI||XbaI||PstI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |1cut(SpeI)||23.5||0µL||0.5µL||0µL||0µL||3µL||3µL||0µL||30µL | ||
| + | |- | ||
| + | |NC||02.3||0µL||0µL||0µL||0µL||1µL||1µL||5.7µL||10µL | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | ! ||9/13 Plux-P ||EcoRI||SpeI||XbaI||PstI|| Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |1cuts(SpeI)||23.8||0µL||0.2µL||0µL||1µL||3µL||3µL||0µL||30µL | ||
| + | |- | ||
| + | |NC||8||0µL||0µL||0µL||0µL||1µL||1µL||0µL||10µL | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | ! ||9/5 RBS-lacZα-DT||EcoRI||SpeI||XbaI||PstI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2cut(XbaI&PstI)||10.8||0µL||0µL||1µL||1µL||3µL||3µL||11.2µL||30µL | ||
| + | |- | ||
| + | |NC||0.5||0µL||0µL||0µL||0µL||1µL||1µL||7.5µL||10µL | ||
| + | |} | ||
| + | |||
| + | ===Electrophoresis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Hirano</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
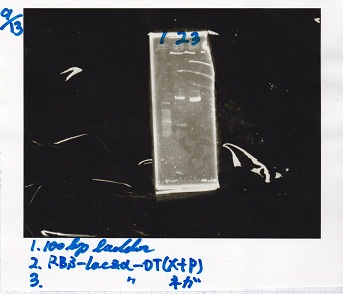
| + | |1||100bp ladder | ||
| + | |- | ||
| + | |2||RBS-lacZα-DT(XbaI&PstI) | ||
| + | |- | ||
| + | |3||RBS-lacZα-DT(NC) | ||
| + | |} | ||
| + | [[File:igku_91313.jpg]]<br> | ||
| + | </div> | ||
| + | |||
| + | ===Ligation=== | ||
| + | |||
| + | <div class="experiment"> | ||
| + | <span class="author">No Name</span> | ||
| + | {| class="wikitable" | ||
| + | !state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | ||
| + | |- | ||
| + | |experiment||9/13 Plac (SpeI&PstI)||8.5µL||9/13 pT181 antisense (XbaI&PstI)||2.1 µL||5.3 µL | ||
| + | |- | ||
| + | |experiment||9/13 Plac (SpeI&PstI)||8.5 µL||9/10 aptamer 12_1R-DT (XbaI&PstI)||1.8 µL||5.15µL | ||
| + | |- | ||
| + | |experiment||9/13 Plac (SpeI&PstI)||8.5 µL||9/2 pT181 attenuator(XbaI&PstI)||2.3 µL||10.4 µL | ||
| + | |- | ||
| + | |experiment||9/13 Plux (SpeI&PstI)||11.4 µL||9/13 RBS-lysis1-DT (XbaI&PstI)||5.0 µL||8.2 µL | ||
| + | |- | ||
| + | |experiment||9/13 Plux (SpeI&PstI)||11.4 µL||9/13 RBS-lysis2-DT (XbaI&PstI)||7.0 µL||9.2 µL | ||
| + | |- | ||
| + | |experiment||9/13 Plux (SpeI&PstI)||7.2 µL||9/13 RBS-lysis3-DT (XbaI&PstI)||6.0 µL||6.6 µL | ||
| + | |- | ||
| + | |experiment||9/13 Pcon-RBS-luxR-DT (EcoRI&XbaI)||2.0 µL||9/4 Plux-RBS-GFP-DT (EcoRI&SpeI)||5.2 µL||3.6 µL | ||
| + | |- | ||
| + | |experiment||9/13 Pcon-RBS-luxR-DT (EcoRI&XbaI)||2.0 µL||9/10 aptamer 12_1R-DT (XbaI&PstI)||1.6 µL||1.8 µL | ||
| + | |- | ||
| + | |experiment||9/12 Ptet-pT181 antisense (--)||1.0 µL||9/10 spinach-DT (--)||2.4 µL||1.7 µL | ||
| + | |} | ||
| + | |||
| + | ===PCR=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||Big Dye||MilliQ||5x buffer||temp(400mg)||SasA_fwd primer||SasA_rev primer||total | ||
| + | |- | ||
| + | |9/7 tetR aptamer 12_1R(pSB1C3) ||0.5||6.05||1.75||1.7||0.5||0||10.5 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||Big Dye||MilliQ||5x buffer||temp(400mg)||SasA_fwd primer||SasA_rev primer||total | ||
| + | |- | ||
| + | |9/6 pT181 antisense-1(pSB1C3) ||0.5||6.05||1.75||1.7||0.5||0||10.5 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||Big Dye||MilliQ||5x buffer||temp(400mg)||SasA_fwd primer||SasA_rev primer||total | ||
| + | |- | ||
| + | |9/6 pT181 attenuator (pSB1C3)||0.5||3.85||1.75||3.9||0.5||0||10.5 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||Big Dye||MilliQ||5x buffer||temp(400mg)||SasA_fwd primer||SasA_rev primer||total | ||
| + | |- | ||
| + | |8/27 Pλ-RBS-luxI-DT-1||0.5||2.55||1.75||5.2||0.5||0||10.5 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||Big Dye||MilliQ||5x buffer||temp(400mg)||SasA_fwd primer||SasA_rev primer||total | ||
| + | |- | ||
| + | |8/31 Pbad/araC-RBS-RFP-2||0.5||3.75||1.75||4||0.5||0||10.5 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||Big Dye||MilliQ||5x buffer||temp(400mg)||SasA_fwd primer||SasA_rev primer||total | ||
| + | |- | ||
| + | |9/13 Plac(pSB1A2) ||0.5||4.2||1.75||3.5||0.5||0||10.5 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||Big Dye||MilliQ||5x buffer||temp(400mg)||SasA_fwd primer||SasA_rev primer||total | ||
| + | |- | ||
| + | |8/20 J23100-RBS-lacZα-DT||0.5||6.35||1.75||1.4||0.5||0||10.5 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||Big Dye||MilliQ||5x buffer||temp(400mg)||SasA_fwd primer||SasA_rev primer||total | ||
| + | |- | ||
| + | |9/7 Ptet-RBS-lacZα-DT||0.5||6.05||1.75||1.7||0.5||0||10.5 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||Big Dye||MilliQ||5x buffer||temp(400mg)||SasA_fwd primer||SasA_rev primer||total | ||
| + | |- | ||
| + | |9/8 J23100-spinach-DT||0.5||5.75||1.75||2||0.5||0||10.5 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||Big Dye||MilliQ||5x buffer||temp(400mg)||SasA_fwd primer||SasA_rev primer||total | ||
| + | |- | ||
| + | |9/11 RBS-lysis1-DT||0.5||6.05||1.75||1.7||0.5||0||10.5 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||Big Dye||MilliQ||5x buffer||temp(400mg)||SasA_fwd primer||SasA_rev primer||total | ||
| + | |- | ||
| + | |9/12 RBS-lysis2-DT||0.5||6.75||1.75||1.0||0.5||0||10.5 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||Big Dye||MilliQ||5x buffer||temp(400mg)||SasA_fwd primer||SasA_rev primer||total | ||
| + | |- | ||
| + | |9/12 RBS-lysis3-DT||0.5||6.75||1.75||1.0||0.5||0||10.5 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||Big Dye||MilliQ||5x buffer||temp(400mg)||SasA_fwd primer||SasA_rev primer||total | ||
| + | |- | ||
| + | |9/8 J23100-spinach-DT||0.5||6.35||1.75||1.4||0||0.5||10.5 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||Big Dye||MilliQ||5x buffer||temp(400mg)||SasA_fwd primer||SasA_rev primer||total | ||
| + | |- | ||
| + | |8/27 Pλ-RBS-luxI-DT-1||0.5||2.55||1.75||5.2||0||0.5||10.5 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||Big Dye||MilliQ||5x buffer||temp(400mg)||SasA_fwd primer||SasA_rev primer||total | ||
| + | |- | ||
| + | |9/12 RBS-lysis2-DT||0.5||6.75||1.75||1.0||0||0.5||10.5 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||Big Dye||MilliQ||5x buffer||temp(400mg)||SasA_fwd primer||SasA_rev primer||total | ||
| + | |- | ||
| + | |9/11 RBS-lysis1-DT||0.5||6.05||1.75||1.7||0||0.5||10.5 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |96°C||96°C||50°C||68°C||-- | ||
| + | |- | ||
| + | |2m||10s||5s||4m||30 | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===PCR=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Yoshida</span> | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||KOD plus||10x buffer||dNTP||MgSO4||Prefix-RBS-RpaB_fwd primer||RpaB-GG4-suffix_rev primer||MilliQ||total | ||
| + | |- | ||
| + | |RpaB||0.5||2.5||2.5||1.5||0.75||0.75||16.5||25 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||KOD plus||10x buffer||dNTP||MgSO4||Prefix-PkaiBC_fwd primer ver.3||PkaiBC-suffix_rev primer ver.3||MilliQ||total | ||
| + | |- | ||
| + | |RpaB||0.5||2.5||2.5||1.5||0.75||0.75||16.5||25 | ||
| + | |} | ||
| + | |||
| + | |||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94°C||98°C||58°C||68°C||-- | ||
| + | |- | ||
| + | |2m||10s||30s||30s||30 | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Gel Extraction=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
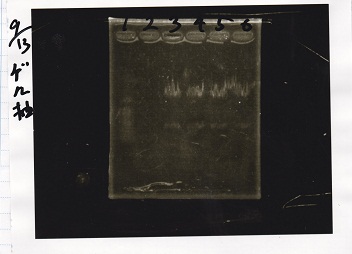
| + | |1||100bp ladder||-- | ||
| + | |- | ||
| + | |3||RBS-lacZα-DT||XbaI&PstI | ||
| + | |- | ||
| + | |4||RBS-lacZα-DT||XbaI&PstI | ||
| + | |- | ||
| + | |5||RBS-lacZα-DT||XbaI&PstI | ||
| + | |- | ||
| + | |6||RBS-lacZα-DT||XbaI&PstI | ||
| + | |} | ||
| + | |||
| + | [[File:igku_91318.jpg]]<br> | ||
| + | [[File:igku_91319.jpg]]<br> | ||
| + | |||
| + | ===Transformation=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Name||Sample||Competent Cells||Total||Plate | ||
| + | |- | ||
| + | |9/13 Plac+pT181 antisense||2µL||20µL||22µL||Amp | ||
| + | |- | ||
| + | |9/13 Plac+apt12_1R||2µL||20µL||22µL||Amp | ||
| + | |- | ||
| + | |9/13 Plac+pT181 attenuator||2µL||20µL||22µL||Amp | ||
| + | |- | ||
| + | |9/13 Plux+pT181 antisense||2µL||20µL||22µL||CP | ||
| + | |- | ||
| + | |9/13 Plux+RBS-lysis2-DT||2µL||20µL||22µL||CP | ||
| + | |- | ||
| + | |9/13 Plux+RBS-lysis2-DT||2µL||20µL||22µL||CP | ||
| + | |- | ||
| + | |9/13 Pcon-RBS-lacR-DT+Plux-RBS-GFP-DT||2µL||20µL||22µL||Amp | ||
| + | |- | ||
| + | |9/13 Pcon-pT181 antisense+apt12_1R-DT||2µL||20µL||22µL||Amp | ||
| + | |- | ||
| + | |9/13 Ptet-pT181antisense+spinach-DT||2µL||20µL||22µL||CP | ||
| + | |- | ||
| + | |Pλ-luxI-1||2µL||20µL||22µL||Amp | ||
| + | |} | ||
| + | </div> | ||
Latest revision as of 20:43, 27 September 2013
Contents |
Sep 13
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 9/12 Fusion6 antisense | 245.9 | 1.88 | 1.41 |
| 9/12 Plux-3 | 115.5 | 1.58 | 1.32 |
| 9/12 Plux-4 | 125.3 | 1.93 | 1.44 |
| 9/12 Fusion3n2 attenuator-1 | 231.9 | 1.81 | 1.31 |
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kb ladder | -- |
| 2 | RBS-lysis1-DT | XbaI&PstI |
| 3 | RBS-lysis1-DT | XbaI&PstI |
| 4 | RBS-lysis1-DT | XbaI&PstI |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kb ladder | -- |
| 3 | RBS-lysis2-DT | XbaI&PstI |
| 4 | RBS-lysis2-DT | XbaI&PstI |
| 5 | RBS-lysis2-DT | XbaI&PstI |
| Lane | DNA | Enzyme |
|---|---|---|
| 3 | RBS-lysis3-DT | XbaI&PstI |
| 4 | RBS-lysis3-DT | XbaI&PstI |
| 5 | RBS-lysis3-DT | XbaI&PstI |
| 6 | 1kb ladder | -- |
| Lane | DNA | Enzyme |
|---|---|---|
| 2 | 1kb ladder | -- |
| 3 | pSB1C3 | EcoRI&SpeI |
| 4 | pSB1C3 | EcoRI&SpeI |
| 5 | pSB1C3 | EcoRI&SpeI |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kb ladder | -- |
| 2 | pSB1C3 | XbaI&PstI |
| 3 | pSB1C3 | XbaI&PstI |
| 4 | pSB1C3 | XbaI&PstI |
Restriction Enzyme Digestion
| 9/13 Plac | EcoRI | SpeI | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 1cuts(PstI) | 16 | 0µL | 0µL | 0µL | 1µL | 3µL | 3µL | 7µL | 30µL |
| NC | 0.8 | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.2µL | 10µL |
| 9/10 Pcon attenuator | EcoRI | SpeI | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 1cut(PstI) | 10 | 0µL | 0µL | 0µL | 1µL | 3µL | 3µL | 13µL | 30µL |
| NC | 0.5 | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL |
| 9/12 Plux | EcoRI | SpeI | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 1cuts(PstI) | 12 | 0µL | 0µL | 0µL | 1µL | 3µL | 3µL | 11µL | 30µL |
| NC | 0.6 | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.4µL | 10µL |
| 9/12 DT-E | EcoRI | SpeI | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 1cut(XbaI) | 23.7 | 0µL | 0µL | 1µL | 0µL | 3µL | 3µL | 0µL | 30.7µL |
| NC | 1.2 | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 6.8µL | 10µL |
| 9/12 Pcon-RBS-luxR-bTE | EcoRI | SpeI | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 1cuts(XbaI) | 22.4 | 0µL | 0µL | 1µL | 0µL | 3µL | 3µL | 0.6µL | 30µL |
| NC | 2.2 | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 5.8µL | 10µL |
| 9/12 Pcon-P | EcoRI | SpeI | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 1cut(SpeI) | 21 | 0µL | 1µL | 0µL | 0µL | 3µL | 3µL | 12.0µL | 30µL |
| NC | 2.1 | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 5.7µL | 10µL |
M9 (EDTA)
1mM EDTA
| volume | 10ml |
|---|---|
| Na2HPO4 | 300mg |
| KH2PO4 | 150mg |
| NaCl | 25mg |
| NH4Cl | 50mg |
| Fe(3)-EDTA | 21mg |
| volume | 10ml |
|---|---|
| Na2HPO4 | 300mg |
| KH2PO4 | 150mg |
| NaCl | 25mg |
| NH4Cl | 50mg |
| Fe(3)-EDTA | 105mg |
</div>
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kb ladder | -- |
| 3 | Pcon-P | SpeI |
| 4 | Pcon-P | SpeI |
| 5 | Pcon-P | SpeI |
Liquid Culture
| Sample | medium |
|---|---|
| 9/11 aptamer12_1M(pSB1C3)-1 | -- |
| 9/11 aptamer 12_P1 | -- |
| 9/11 Fusaion1 antisense-4 | -- |
| 9/12 pSB4K5-1 | -- |
| 9/12 pSB4K5-2 | -- |
Restriction Enzyme Digestion
| 9/13 Plac-P | EcoRI | SpeI | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 1cuts(SpeI) | 23.5 | 0µL | 0.5µL | 0µL | 0µL | 3µL | 3µL | 0µL | 30µL |
| NC | 8 | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 0µL | 10µL |
| 9/13 Pcon-pT181 attenuator-P | EcoRI | SpeI | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 1cut(SpeI) | 23.5 | 0µL | 0.5µL | 0µL | 0µL | 3µL | 3µL | 0µL | 30µL |
| NC | 02.3 | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 5.7µL | 10µL |
| 9/13 Plux-P | EcoRI | SpeI | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 1cuts(SpeI) | 23.8 | 0µL | 0.2µL | 0µL | 1µL | 3µL | 3µL | 0µL | 30µL |
| NC | 8 | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 0µL | 10µL |
| 9/5 RBS-lacZα-DT | EcoRI | SpeI | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2cut(XbaI&PstI) | 10.8 | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 11.2µL | 30µL |
| NC | 0.5 | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL |
Electrophoresis
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | 9/13 Plac (SpeI&PstI) | 8.5µL | 9/13 pT181 antisense (XbaI&PstI) | 2.1 µL | 5.3 µL |
| experiment | 9/13 Plac (SpeI&PstI) | 8.5 µL | 9/10 aptamer 12_1R-DT (XbaI&PstI) | 1.8 µL | 5.15µL |
| experiment | 9/13 Plac (SpeI&PstI) | 8.5 µL | 9/2 pT181 attenuator(XbaI&PstI) | 2.3 µL | 10.4 µL |
| experiment | 9/13 Plux (SpeI&PstI) | 11.4 µL | 9/13 RBS-lysis1-DT (XbaI&PstI) | 5.0 µL | 8.2 µL |
| experiment | 9/13 Plux (SpeI&PstI) | 11.4 µL | 9/13 RBS-lysis2-DT (XbaI&PstI) | 7.0 µL | 9.2 µL |
| experiment | 9/13 Plux (SpeI&PstI) | 7.2 µL | 9/13 RBS-lysis3-DT (XbaI&PstI) | 6.0 µL | 6.6 µL |
| experiment | 9/13 Pcon-RBS-luxR-DT (EcoRI&XbaI) | 2.0 µL | 9/4 Plux-RBS-GFP-DT (EcoRI&SpeI) | 5.2 µL | 3.6 µL |
| experiment | 9/13 Pcon-RBS-luxR-DT (EcoRI&XbaI) | 2.0 µL | 9/10 aptamer 12_1R-DT (XbaI&PstI) | 1.6 µL | 1.8 µL |
| experiment | 9/12 Ptet-pT181 antisense (--) | 1.0 µL | 9/10 spinach-DT (--) | 2.4 µL | 1.7 µL |
PCR
| genome DNA | Big Dye | MilliQ | 5x buffer | temp(400mg) | SasA_fwd primer | SasA_rev primer | total |
|---|---|---|---|---|---|---|---|
| 9/7 tetR aptamer 12_1R(pSB1C3) | 0.5 | 6.05 | 1.75 | 1.7 | 0.5 | 0 | 10.5 |
| genome DNA | Big Dye | MilliQ | 5x buffer | temp(400mg) | SasA_fwd primer | SasA_rev primer | total |
|---|---|---|---|---|---|---|---|
| 9/6 pT181 antisense-1(pSB1C3) | 0.5 | 6.05 | 1.75 | 1.7 | 0.5 | 0 | 10.5 |
| genome DNA | Big Dye | MilliQ | 5x buffer | temp(400mg) | SasA_fwd primer | SasA_rev primer | total |
|---|---|---|---|---|---|---|---|
| 9/6 pT181 attenuator (pSB1C3) | 0.5 | 3.85 | 1.75 | 3.9 | 0.5 | 0 | 10.5 |
| genome DNA | Big Dye | MilliQ | 5x buffer | temp(400mg) | SasA_fwd primer | SasA_rev primer | total |
|---|---|---|---|---|---|---|---|
| 8/27 Pλ-RBS-luxI-DT-1 | 0.5 | 2.55 | 1.75 | 5.2 | 0.5 | 0 | 10.5 |
| genome DNA | Big Dye | MilliQ | 5x buffer | temp(400mg) | SasA_fwd primer | SasA_rev primer | total |
|---|---|---|---|---|---|---|---|
| 8/31 Pbad/araC-RBS-RFP-2 | 0.5 | 3.75 | 1.75 | 4 | 0.5 | 0 | 10.5 |
| genome DNA | Big Dye | MilliQ | 5x buffer | temp(400mg) | SasA_fwd primer | SasA_rev primer | total |
|---|---|---|---|---|---|---|---|
| 9/13 Plac(pSB1A2) | 0.5 | 4.2 | 1.75 | 3.5 | 0.5 | 0 | 10.5 |
| genome DNA | Big Dye | MilliQ | 5x buffer | temp(400mg) | SasA_fwd primer | SasA_rev primer | total |
|---|---|---|---|---|---|---|---|
| 8/20 J23100-RBS-lacZα-DT | 0.5 | 6.35 | 1.75 | 1.4 | 0.5 | 0 | 10.5 |
| genome DNA | Big Dye | MilliQ | 5x buffer | temp(400mg) | SasA_fwd primer | SasA_rev primer | total |
|---|---|---|---|---|---|---|---|
| 9/7 Ptet-RBS-lacZα-DT | 0.5 | 6.05 | 1.75 | 1.7 | 0.5 | 0 | 10.5 |
| genome DNA | Big Dye | MilliQ | 5x buffer | temp(400mg) | SasA_fwd primer | SasA_rev primer | total |
|---|---|---|---|---|---|---|---|
| 9/8 J23100-spinach-DT | 0.5 | 5.75 | 1.75 | 2 | 0.5 | 0 | 10.5 |
| genome DNA | Big Dye | MilliQ | 5x buffer | temp(400mg) | SasA_fwd primer | SasA_rev primer | total |
|---|---|---|---|---|---|---|---|
| 9/11 RBS-lysis1-DT | 0.5 | 6.05 | 1.75 | 1.7 | 0.5 | 0 | 10.5 |
| genome DNA | Big Dye | MilliQ | 5x buffer | temp(400mg) | SasA_fwd primer | SasA_rev primer | total |
|---|---|---|---|---|---|---|---|
| 9/12 RBS-lysis2-DT | 0.5 | 6.75 | 1.75 | 1.0 | 0.5 | 0 | 10.5 |
| genome DNA | Big Dye | MilliQ | 5x buffer | temp(400mg) | SasA_fwd primer | SasA_rev primer | total |
|---|---|---|---|---|---|---|---|
| 9/12 RBS-lysis3-DT | 0.5 | 6.75 | 1.75 | 1.0 | 0.5 | 0 | 10.5 |
| genome DNA | Big Dye | MilliQ | 5x buffer | temp(400mg) | SasA_fwd primer | SasA_rev primer | total |
|---|---|---|---|---|---|---|---|
| 9/8 J23100-spinach-DT | 0.5 | 6.35 | 1.75 | 1.4 | 0 | 0.5 | 10.5 |
| genome DNA | Big Dye | MilliQ | 5x buffer | temp(400mg) | SasA_fwd primer | SasA_rev primer | total |
|---|---|---|---|---|---|---|---|
| 8/27 Pλ-RBS-luxI-DT-1 | 0.5 | 2.55 | 1.75 | 5.2 | 0 | 0.5 | 10.5 |
| genome DNA | Big Dye | MilliQ | 5x buffer | temp(400mg) | SasA_fwd primer | SasA_rev primer | total |
|---|---|---|---|---|---|---|---|
| 9/12 RBS-lysis2-DT | 0.5 | 6.75 | 1.75 | 1.0 | 0 | 0.5 | 10.5 |
| genome DNA | Big Dye | MilliQ | 5x buffer | temp(400mg) | SasA_fwd primer | SasA_rev primer | total |
|---|---|---|---|---|---|---|---|
| 9/11 RBS-lysis1-DT | 0.5 | 6.05 | 1.75 | 1.7 | 0 | 0.5 | 10.5 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 96°C | 96°C | 50°C | 68°C | -- |
| 2m | 10s | 5s | 4m | 30 |
PCR
| genome DNA | KOD plus | 10x buffer | dNTP | MgSO4 | Prefix-RBS-RpaB_fwd primer | RpaB-GG4-suffix_rev primer | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| RpaB | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 16.5 | 25 |
| genome DNA | KOD plus | 10x buffer | dNTP | MgSO4 | Prefix-PkaiBC_fwd primer ver.3 | PkaiBC-suffix_rev primer ver.3 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| RpaB | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 16.5 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 58°C | 68°C | -- |
| 2m | 10s | 30s | 30s | 30 |
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 3 | RBS-lacZα-DT | XbaI&PstI |
| 4 | RBS-lacZα-DT | XbaI&PstI |
| 5 | RBS-lacZα-DT | XbaI&PstI |
| 6 | RBS-lacZα-DT | XbaI&PstI |
Transformation
| Name | Sample | Competent Cells | Total | Plate |
|---|---|---|---|---|
| 9/13 Plac+pT181 antisense | 2µL | 20µL | 22µL | Amp |
| 9/13 Plac+apt12_1R | 2µL | 20µL | 22µL | Amp |
| 9/13 Plac+pT181 attenuator | 2µL | 20µL | 22µL | Amp |
| 9/13 Plux+pT181 antisense | 2µL | 20µL | 22µL | CP |
| 9/13 Plux+RBS-lysis2-DT | 2µL | 20µL | 22µL | CP |
| 9/13 Plux+RBS-lysis2-DT | 2µL | 20µL | 22µL | CP |
| 9/13 Pcon-RBS-lacR-DT+Plux-RBS-GFP-DT | 2µL | 20µL | 22µL | Amp |
| 9/13 Pcon-pT181 antisense+apt12_1R-DT | 2µL | 20µL | 22µL | Amp |
| 9/13 Ptet-pT181antisense+spinach-DT | 2µL | 20µL | 22µL | CP |
| Pλ-luxI-1 | 2µL | 20µL | 22µL | Amp |
 "
"