Team:Shenzhen BGIC ATCG/results
From 2013.igem.org
| Line 153: | Line 153: | ||
<ul id="myGallery1"> | <ul id="myGallery1"> | ||
| - | <li><img src="https://static.igem.org/mediawiki/ | + | <li><img src="https://static.igem.org/mediawiki/2013/4/41/Clb6.jpg" title="G1 Phase Promoter" description="clb6 promoter for G1 phase has been verified." /></li> |
| + | <li><img src="https://static.igem.org/mediawiki/2013/6/6e/Target2.jpg" title="Reporter Targeting" description="Test for targeting peptides to mitochondria, nucleus and vacuolar. "/></li> | ||
| + | <li><img src="https://static.igem.org/mediawiki/igem.org/8/86/Flo2.jpg" title="Degradation Tags" description="Degradation tags testing device for E. coli."/></li> | ||
| + | <li><img src="https://static.igem.org/mediawiki/2013/a/a8/Degmicro.jpg" title="Microfluidics" description="Microfluidics device for degradation test."/></li> | ||
| + | |||
</ul></div> | </ul></div> | ||
<div id="box1" class="box"> | <div id="box1" class="box"> | ||
<h3>The Magic</h3> | <h3>The Magic</h3> | ||
<p>Our project “Cell Magic", a complex work has been completed partly,due to the time limitation. The results we have gotten are listed below:</p> | <p>Our project “Cell Magic", a complex work has been completed partly,due to the time limitation. The results we have gotten are listed below:</p> | ||
| - | <p> We verified the | + | <p>We verified some of the cyclin promoters. The clb6 promoter, which is supposed to be expressed in G1 phase, has been verified perfectly.</p> |
| - | + | <img src="https://static.igem.org/mediawiki/2013/4/41/Clb6.jpg" title="G1 Phase Promoter" description="clb6 promoter for G1 phase has been verified." /> | |
| + | <p>Targeting peptides to three locations have been verified. Others are still being constructed and tested.</p> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/6/6e/Target2.jpg" title="Reporter Targeting" description="Test for targeting peptides to mitochondria, nucleus and vacuolar. "/> | ||
| + | <p>We got good data for degradation tags for <i>E. coli</i>. Construction of degradation devices for yeast have been finished and being tested.</p> | ||
| + | <img src="https://static.igem.org/mediawiki/igem.org/8/86/Flo2.jpg" /> | ||
| + | <p>Microfluidics device has been successfully developed for measurement and cell synchronization.</p> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/a/a8/Degmicro.jpg" /> | ||
| + | <p>Cell synchronization device and alternative splicing device are still in construction, related results will be updated later.</p> | ||
<video src="https://static.igem.org/mediawiki/2013/c/cc/Microfluidic.mp4" controls="controls" /> | <video src="https://static.igem.org/mediawiki/2013/c/cc/Microfluidic.mp4" controls="controls" /> | ||
| Line 168: | Line 179: | ||
<div class="media"> | <div class="media"> | ||
<ul id="myGallery2"> | <ul id="myGallery2"> | ||
| - | <li><img src="https://static.igem.org/mediawiki/ | + | <li><img src="https://static.igem.org/mediawiki/2013/4/41/Clb6.jpg" title="G1 Phase Promoter" description="clb6 promoter for G1 phase has been verified." /></li> |
</ul></div> | </ul></div> | ||
<div id="box2" class="box"> | <div id="box2" class="box"> | ||
| Line 185: | Line 196: | ||
<div class="media"> | <div class="media"> | ||
<ul id="myGallery3"> | <ul id="myGallery3"> | ||
| - | <li><img src="https://static.igem.org/mediawiki/ | + | <li><img src="https://static.igem.org/mediawiki/2013/6/6e/Target2.jpg" title="Reporter Targeting" description="Test for targeting peptides to mitochondria, nucleus and vacuolar. "/></li> |
</ul></div> | </ul></div> | ||
<div id="box3" class="box"> | <div id="box3" class="box"> | ||
| Line 199: | Line 210: | ||
<div class="media"> | <div class="media"> | ||
<ul id="myGallery4"> | <ul id="myGallery4"> | ||
| - | <li><img src="https://static.igem.org/mediawiki/ | + | <li><img src="https://static.igem.org/mediawiki/igem.org/8/86/Flo2.jpg" title="E. coli Version" description="Degradation tags testing device for E. coli"/></li> |
</ul></div> | </ul></div> | ||
<div id="box4" class="box"> | <div id="box4" class="box"> | ||
| Line 220: | Line 231: | ||
<img src="https://static.igem.org/mediawiki/igem.org/8/86/Flo2.jpg" /> | <img src="https://static.igem.org/mediawiki/igem.org/8/86/Flo2.jpg" /> | ||
<p>The test results for K1051258 and positive control. A. K1051258 in bright field; B. K1051258 in exciting lights; C. Positive control in bright field; D. K1051258 in exciting lights.</p> | <p>The test results for K1051258 and positive control. A. K1051258 in bright field; B. K1051258 in exciting lights; C. Positive control in bright field; D. K1051258 in exciting lights.</p> | ||
| - | <p>In picture, there are only obvious lights in the picture B, indicated the degradation rates are working</p> | + | <p>In picture, there are only obvious lights in the picture B, indicated the degradation rates are working.</p> |
<p>预留酶标仪结果图和柱状图,降解效率换算</p> | <p>预留酶标仪结果图和柱状图,降解效率换算</p> | ||
<p>Enzyme - labelled meter detect the fluorescent protein intensity</p> | <p>Enzyme - labelled meter detect the fluorescent protein intensity</p> | ||
| Line 230: | Line 241: | ||
--------------------> | --------------------> | ||
<img src="https://static.igem.org/mediawiki/2013/a/a8/Degmicro.jpg" /> | <img src="https://static.igem.org/mediawiki/2013/a/a8/Degmicro.jpg" /> | ||
| - | <p>The test results of BBa_K1051258 in chip. A,LB medium,O minuts; B, IPTG medium,9minutes; C,IPTG medium, 15 minutes | + | <p>The test results of BBa_K1051258 in chip. A,LB medium,O minuts; B, IPTG medium,9minutes; C,IPTG medium, 15 minutes</p> |
<p>First of all, yeast will be measured after shaking to about OD2.0 (600)based on the chip which was washed by plasma water, vacuum pumping. The bacteria liquid was pushed into the chip, letting the cells enter the small triangle. Then use the constant flow pump culture medium into chip (the laboratory constant temperature, can not guarantee the training environment, 37 ° E. coli slower growth). The medium speed is about 200ul/h. Finally we test the data after yeast fulled in the triangles.</p> | <p>First of all, yeast will be measured after shaking to about OD2.0 (600)based on the chip which was washed by plasma water, vacuum pumping. The bacteria liquid was pushed into the chip, letting the cells enter the small triangle. Then use the constant flow pump culture medium into chip (the laboratory constant temperature, can not guarantee the training environment, 37 ° E. coli slower growth). The medium speed is about 200ul/h. Finally we test the data after yeast fulled in the triangles.</p> | ||
<p>Using the IPTG medium, the new RFP expression was stopped and we can regard the lights as degradation tags'effecience. Obviously, the lights are decreasing along with the time. Thus, it indicates that the degradation tags work effectively</p> | <p>Using the IPTG medium, the new RFP expression was stopped and we can regard the lights as degradation tags'effecience. Obviously, the lights are decreasing along with the time. Thus, it indicates that the degradation tags work effectively</p> | ||
Revision as of 21:04, 27 September 2013


Playing with my eyes
aren't you?
Hi I am Dr. Mage!
A "budding" yeast cell!
The Magic
Our project “Cell Magic", a complex work has been completed partly,due to the time limitation. The results we have gotten are listed below:
We verified some of the cyclin promoters. The clb6 promoter, which is supposed to be expressed in G1 phase, has been verified perfectly.

Targeting peptides to three locations have been verified. Others are still being constructed and tested.

We got good data for degradation tags for E. coli. Construction of degradation devices for yeast have been finished and being tested.

Microfluidics device has been successfully developed for measurement and cell synchronization.

Cell synchronization device and alternative splicing device are still in construction, related results will be updated later.
Promoter Verification
Using the GFP as reporter and morphological alteration as cell cycle representation, we verified the Clb6 can be activated in G1 phase in the yeast.
As shown in the picture in normal lights, there are some yeast were budding. These budded yeast cells and small size budding yeasts (within the red circles) are assumed in the G1 phase of cell cycle. And the next picture was taken under activation light, thus the green lights can representing the Clb6 promoter expressing. The two pictures indicated the Clb6 were expressed in the G1 phase as we expected when designing the experiments
PS: we modified the contrast ratio to lower the lights of neighbor cells, thus our results looking better.

预留橙色cln3图
预留流式细胞仪图
Reporter Locating
Fluorescence proteins have been modified to RFC[23], and stop codons have been removed for fusion protein purpose.

The four pictures shows the reporters can rightly locate to Mitochondria, Nucleus and Vacuole,respectively. Picture A is the negative control, all yeast cells are lighted with GFP. And figure B is the reporter to the mitochondria, we can saw there are several light spots in one cells. Figure C is the reporter located in nucleus, the green spots are small and there is only one in a yeast cell.The last picture shows the reporter of Vacuolar membrane, the green lights were discrete in cells which was as expected.

Degradation Rate
In E.coli, the adaptor SspB tethers ssrAtagged substrates to the ClpXP protease, causing a modest increase in their rate of degradation. Which means, a variation of the WT SsrA tag sequence (K1051206, K1051207 and K1051208) will accelerate the degradation of proteins when fused to their C-terminal. Thus the degradation rates are dependent on concentration of proteases and binding mediators.
We constructed the measurement pathway of each tag (K1051257, K1051258 and K1051259) to test the rates of degradation of tagged proteins respectively. J04450 was used as positive control because of the same promoter and fluorescent protein.
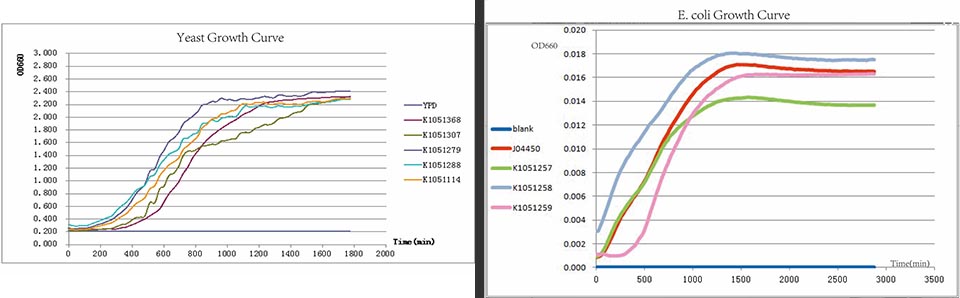
In every curcuits measurement, we firstly test the growth curve through detecting the absorbance. Taken small amount of bateria, inoculate into 400ul LB medium, ensuring the initial concentration between 0.02-0.05 (OD600). Cultured in the room temperature, detect the OD600 value every 20 minutes. Y axis represents the logarithm of bacteria number, X axis represents the growth time.

From right to left, the negative control,BBa_K1051257, BBa_K1051258, BBa_K1051259, J04450 as Positive Control. As the pictures showed, the lights of RFP within three degradation tags are decreasing.

The test results for K1051258 and positive control. A. K1051258 in bright field; B. K1051258 in exciting lights; C. Positive control in bright field; D. K1051258 in exciting lights.
In picture, there are only obvious lights in the picture B, indicated the degradation rates are working.
预留酶标仪结果图和柱状图,降解效率换算
Enzyme - labelled meter detect the fluorescent protein intensity
First of all, take a certain amount of bacteria liquid, recovery to around OD0.6(600), ensuring the bacteria in the logarithmic growth phase. Diluted and then the bacteria transferred to 96 well plates, measured their fluorescence intensity. Red fluorescent protein using an excitation wavelength of 584nm, and its emission wavelength is 607nm.When measuring, we first detect the OD600 of each strain, removing the factor of bacteria number difference. Thus the fluorescence intensity cannot be altered by bacteria quantity. Then the measurement mode switching for measurement of fluorescence, fluorescence intensity.

The test results of BBa_K1051258 in chip. A,LB medium,O minuts; B, IPTG medium,9minutes; C,IPTG medium, 15 minutes
First of all, yeast will be measured after shaking to about OD2.0 (600)based on the chip which was washed by plasma water, vacuum pumping. The bacteria liquid was pushed into the chip, letting the cells enter the small triangle. Then use the constant flow pump culture medium into chip (the laboratory constant temperature, can not guarantee the training environment, 37 ° E. coli slower growth). The medium speed is about 200ul/h. Finally we test the data after yeast fulled in the triangles.
Using the IPTG medium, the new RFP expression was stopped and we can regard the lights as degradation tags'effecience. Obviously, the lights are decreasing along with the time. Thus, it indicates that the degradation tags work effectively
预留半衰期换算
Cell Synchronization
We made the chip as a platform for watching and synchronize the cells. First step is capture the cells by the chip. As showed in the two figures, both E.coli and budding yeast can be captured by the chip successfully.


Seven phosphorylation sites are mutated by PCR to make Sic1 mutant. However, it has been taking us too long time that we have been still working on right clones. Experiments results will be updated later.
Alternative Splicing
In our project, we attempt to use intron as a switch. However, according to previous research, there are two splicing forms of SRC1 intron: one is complete splicing (5’S) while the other can leave 4bp at its 5’ end (5’L). The remaining 4-bp causes frame-shift and a stop codon in the region of adaptor, thus the following exon will not be translated. However, this kind of switch is not complete – the ratio of 5’L and 5’S varied between 40-60 and 85-15.
In order to make a complete switch, we abandoned the remaining 4bp of SRC1 intron to avoid producing 5’L mRNA. So the result comes out to be: when Hub1p are expressed, they bind to spliceosome to modify it, resulting to easy recognizing 5’S splice site and its splicing; when inhibit the expression of HUB1 by CRISPRi system, spliceosome can hardly recognize 5’S splice site, therefore no intron can be spliced.
However, things didn’t go as we expected. Our experiment showed that this 4bp plays important role in SRC1 intron splicing. So, to achieve our goal, we redesigned another three versions of SRC1 intron:
1. Intron with GG at its 5’ end
2. Intron with CGG at its 5’ end
3. Intron with its original 6bp at both end
However, due to the lack of time, we could not connect all these parts to our test parts.
 "
"
