Template:Kyoto/Notebook/Sep 27
From 2013.igem.org
(Difference between revisions)
(→Gel Extraction) |
(→cDNA Synthesis) |
||
| (37 intermediate revisions not shown) | |||
| Line 48: | Line 48: | ||
|1||1kbp ladder||-- | |1||1kbp ladder||-- | ||
|- | |- | ||
| - | |3||pSB4K5|| | + | |3||pSB4K5||EcoRI+PstI |
|- | |- | ||
| - | |4||pSB4K5|| | + | |4||pSB4K5||EcoRI+PstI |
|- | |- | ||
| - | |5||pSB4K5|| | + | |5||pSB4K5||EcoRI+PstI |
|- | |- | ||
| - | |7||pSB6A1|| | + | |7||pSB6A1||EcoRI+PstI |
|- | |- | ||
| - | |8||pSB6A1|| | + | |8||pSB6A1||EcoRI+PstI |
|- | |- | ||
| - | |9||pSB6A1|| | + | |9||pSB6A1||EcoRI+PstI |
|- | |- | ||
|} | |} | ||
[[File:igku_9274.jpg]]<br> | [[File:igku_9274.jpg]]<br> | ||
[[File:igku_9275.jpg]]<br> | [[File:igku_9275.jpg]]<br> | ||
| - | + | </div> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
===Electrophoresis=== | ===Electrophoresis=== | ||
| Line 89: | Line 82: | ||
|- | |- | ||
|} | |} | ||
| - | + | <br> | |
{| class="wikitable" | {| class="wikitable" | ||
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
| Line 106: | Line 99: | ||
|- | |- | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku_0927_G5.jpg]]<br> |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
</div> | </div> | ||
| Line 219: | Line 135: | ||
|experiment||9/27 pSB4K5(EcoRI+PstI) 12.9ng/µL||7.8µL||9/25 RBS-GFP-DT(XbaI+PstI) 13.9ng/µL||12µL||3.5 µL | |experiment||9/27 pSB4K5(EcoRI+PstI) 12.9ng/µL||7.8µL||9/25 RBS-GFP-DT(XbaI+PstI) 13.9ng/µL||12µL||3.5 µL | ||
|- | |- | ||
| - | |experiment||9/8 pSB4K5 (EcoRI+SpeI) 17.6ng/µL|| | + | |experiment||9/8 pSB4K5 (EcoRI+SpeI) 17.6ng/µL||5.7 µL||8/21 Pcon-GFP-DT(EcoRI+SpeI) 11ng/µL||12.6µL||3.5 µL |
|} | |} | ||
</div> | </div> | ||
| Line 310: | Line 226: | ||
|- | |- | ||
|attenuator(1C3)||--||2||--||20||22 | |attenuator(1C3)||--||2||--||20||22 | ||
| + | |} | ||
| + | |||
| + | ===Miniprep=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {|class="wikitable" | ||
| + | !DNA||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |Pcon-RBS-GFP-DT||137.6||1.95||1.78 | ||
| + | |- | ||
| + | |RBS-GFP-DT||77.0||2.06||1.96 | ||
| + | |- | ||
| + | |Pcon-PT181 attenuator||231.0||1.89||1.89 | ||
| + | |- | ||
| + | |Pcon-antisense-Spinach-DT(2)||130.0||1.56||1.62 | ||
| + | |- | ||
| + | |Pcon-aptamer-DT||218.8||1.92||1.72 | ||
| + | |- | ||
| + | |Pcon-attenuator-DT||180.7||1.95||1.78 | ||
| + | |- | ||
| + | |Pcon-Spinach-DT||177.6||1.96||1.06 | ||
| + | |- | ||
| + | |Pcon-antisense-Spinach-DT(1)||181.1||1.96||1.92 | ||
| + | |- | ||
| + | |Pcon-antisense||152.9||2.02||1.96 | ||
| + | |- | ||
| + | |Pcon-RBS+ezR-DT||164.1||1.93||1.91 | ||
|} | |} | ||
</div> | </div> | ||
| + | |||
| + | ===Restriction Enzyme Digestion=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | ! ||9/27 RBS-GFP-DT||XbaI||PstI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||12µL||1µL||1µL||3µL||3µL||20µL||40µL | ||
| + | |- | ||
| + | |NC||1.3µL||0µL||0µL||1µL||1µL||6.7µL||10µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||9/27 Pcon-tetR-DT||EcoRI||PstI||Buffer||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||12.2µL||1µL||1µL||3µL||12.8µL|| 30µL | ||
| + | |- | ||
| + | |NC||0.6µL||0µL||0µL||1µL||8.4µL||10µL | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===RNA Extraction=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto</span> | ||
| + | {| class="wikitable" | ||
| + | !Name||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |9/27 Pcon-RBS-GFP-DT||686.6||1.99||1.34 | ||
| + | |- | ||
| + | |9/27 Pcon-RBS-GFP-DT (+High salt)||127.6||1.76||0.27 | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===DNA sythesis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||DNA volume||genome DNA wipeout buffer||RNase freewater||RT||RT buffer||Primer Mix||total | ||
| + | |- | ||
| + | |9/27 Pcon-RBS-GFP-DT||0.8µL||1µL||5.2µL ||0.5µL ||2µL||0.5µL ||10µL | ||
| + | |- | ||
| + | |9/27 pcon-RBS-GFP-DT(+high salt)||2.5µL||1µL||3.5µL ||0.5µL ||2µL ||0.5µL ||10µL | ||
| + | |- | ||
| + | |9/27 Pcon-RBS-GFP-DT(revised concentration)|| 0.5µL||1µL||5.5µL||0.5µL||2µL||0.5µL||10µL | ||
| + | |} | ||
| + | </div> | ||
| + | ===Electrophoresis=== | ||
| + | |||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||1kb ladder | ||
| + | |- | ||
| + | |2||Pcon-RBS-GFP-DT | ||
| + | |- | ||
| + | |3|| Pcon-RBS-GFP-DT | ||
| + | |} | ||
| + | [[File:igku_0927_E2.jpg]]<br> | ||
| + | </div> | ||
| + | |||
| + | ===Liquid Culture=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||medium | ||
| + | |- | ||
| + | |Pcon spinach DT ||plusgrow 2µL(Amp) | ||
| + | |- | ||
| + | |Pcon aptamer DT ||plusgrow 2µL(Amp) | ||
| + | |- | ||
| + | |Pcon antisense Spinach DT ||plusgrow 2µL(Amp) | ||
| + | |- | ||
| + | |Spinach DT||plusgrow 4µL(CP) | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===RT PCR=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||KOD plus||10x buffer for KOD-plus-ver.2||2nM dNTPs||25mM MgSO4||F primer||R primer||MilliQ||total | ||
| + | |- | ||
| + | | 0.5||1||2.5||2.5||1.5||0.75||0.75||15.5||25 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | | 94°C|| 98°C|| 49.8°C|| 68°C||-- | ||
| + | |- | ||
| + | |2min ||10sec ||30sec ||10sec ||30 | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Electrophoresis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||Pcon-GFP-DT(GFP) | ||
| + | |- | ||
| + | |2||Pcon-GFP-DT(internal) | ||
| + | |- | ||
| + | |3||Pcon-GFP-DT(HS)(GFP) | ||
| + | |- | ||
| + | |4||Pcon-GFP-DT(internal) | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||Pcon-GFP-DT(GFP) | ||
| + | |- | ||
| + | |2||Pcon-GFP-DT(internal) | ||
| + | |- | ||
| + | |3||100bp ladder | ||
| + | |} | ||
| + | [[File:igku_927E4.jpg]]<br> | ||
| + | </div> | ||
| + | |||
| + | ===Digesting cut check=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||RBS-GFP-DT(XbaI&PstI) | ||
| + | |- | ||
| + | |2||RBS-GFP-DT NC(XbaI&PstI) | ||
| + | |- | ||
| + | |3||1kbp ladder | ||
| + | |- | ||
| + | |4||Pcon-RBS-tetR-DT(EcoRI&SpeI) | ||
| + | |- | ||
| + | |5||Pcon-RBS-tetR-DT NC(EcoRI&SpeI) | ||
| + | |} | ||
| + | [[File:igku_927_E3.jpg]]<br> | ||
| + | </div> | ||
| + | ===Gel Extraction=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||1kbp ladder||-- | ||
| + | |- | ||
| + | |3||RBS-GFP-DT||Xbal+PstI | ||
| + | |- | ||
| + | |4||RBS-GFP-DT||Xbal+PstI | ||
| + | |- | ||
| + | |5||RBS-GFP-DT||Xbal+PstI | ||
| + | |- | ||
| + | |7||1kbp ladder||-- | ||
| + | |- | ||
| + | |9||Pcon-tetR-DT||EcoRI+SpeI | ||
| + | |- | ||
| + | |10||Pcon-tetR-DT||EcoRI+SpeI | ||
| + | |- | ||
| + | |11||Pcon-tetR-DT||EcoRI+SpeI | ||
| + | |- | ||
| + | |} | ||
| + | [[File:igku_0927_G7.jpg]]<br> | ||
| + | [[File:igku_0927_G8.jpg]]<br> | ||
| + | [[File:igku_0927_G9.jpg]]<br> | ||
| + | [[File:igku_0927_G10.jpg]]<br> | ||
| + | |||
| + | ===RNA Extraction=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto</span> | ||
| + | {| class="wikitable" | ||
| + | !||0||5min||15min||20min||23min||28min||33min||36min | ||
| + | |- | ||
| + | |aptamer generator||0.348||0.408||0.550||0.865|||||||| | ||
| + | |- | ||
| + | |Pcon-spinach-DT||0.250||0.240||0.305||0.426||0.471||0.554||0.668||0 | ||
| + | |- | ||
| + | |GFP generator||0.389||0.420||0.516||0.884|||||||| | ||
| + | |- | ||
| + | |antisense-spinach||0.371||0.438||0.537||0.733|||||||| | ||
| + | |- | ||
| + | |Spinach-DT||0.084||0.110||0.134||0.433||0.269||0.326||0.409||0.742 | ||
| + | |- | ||
| + | |} | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | !Name||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |Spinach-DT||83.9||1.63||0.17 | ||
| + | |- | ||
| + | |Pcon-tetRaptamer-DT||425.1||2.10||0.64 | ||
| + | |- | ||
| + | |Pcon-RBS-GFP-DT||262.4||2.01||0.84 | ||
| + | |- | ||
| + | |Pcon-antisense-Spinach-DT||489.1||2.05||0.68 | ||
| + | |- | ||
| + | |Pcon-Spinach-DT||266.6||2.01||0.66 | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===cDNA Synthesis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto</span> | ||
| + | {| class="wikitable" | ||
| + | !||9/27 Spinach-DT||9/23 Pcon-tetRaptamer-DT||9/23 Pcon-RBS-GFP-DT||9/23 Pcon-antisense-spinach-DT||9/23 Pcon-Spinach-DT | ||
| + | |- | ||
| + | |RNA||1||1||1||1||1 | ||
| + | |- | ||
| + | |gDNA wipeout||6||1.2||1.9||1.0||1.9 | ||
| + | |- | ||
| + | |RNase-free water||0||4.8||4.1||5.0||4.1 | ||
| + | |- | ||
| + | |RT||0.5||0.5||0.5||0.5||0.5 | ||
| + | |- | ||
| + | |RT Buffer||2||2||2||2||2 | ||
| + | |- | ||
| + | |Primer mix||0.5||0.5||0.5||0.5||0.5 | ||
| + | |- | ||
| + | |total||10µL||10µL||10µL||10µL||10µL | ||
| + | |} | ||
Latest revision as of 03:47, 28 September 2013
Sep 27
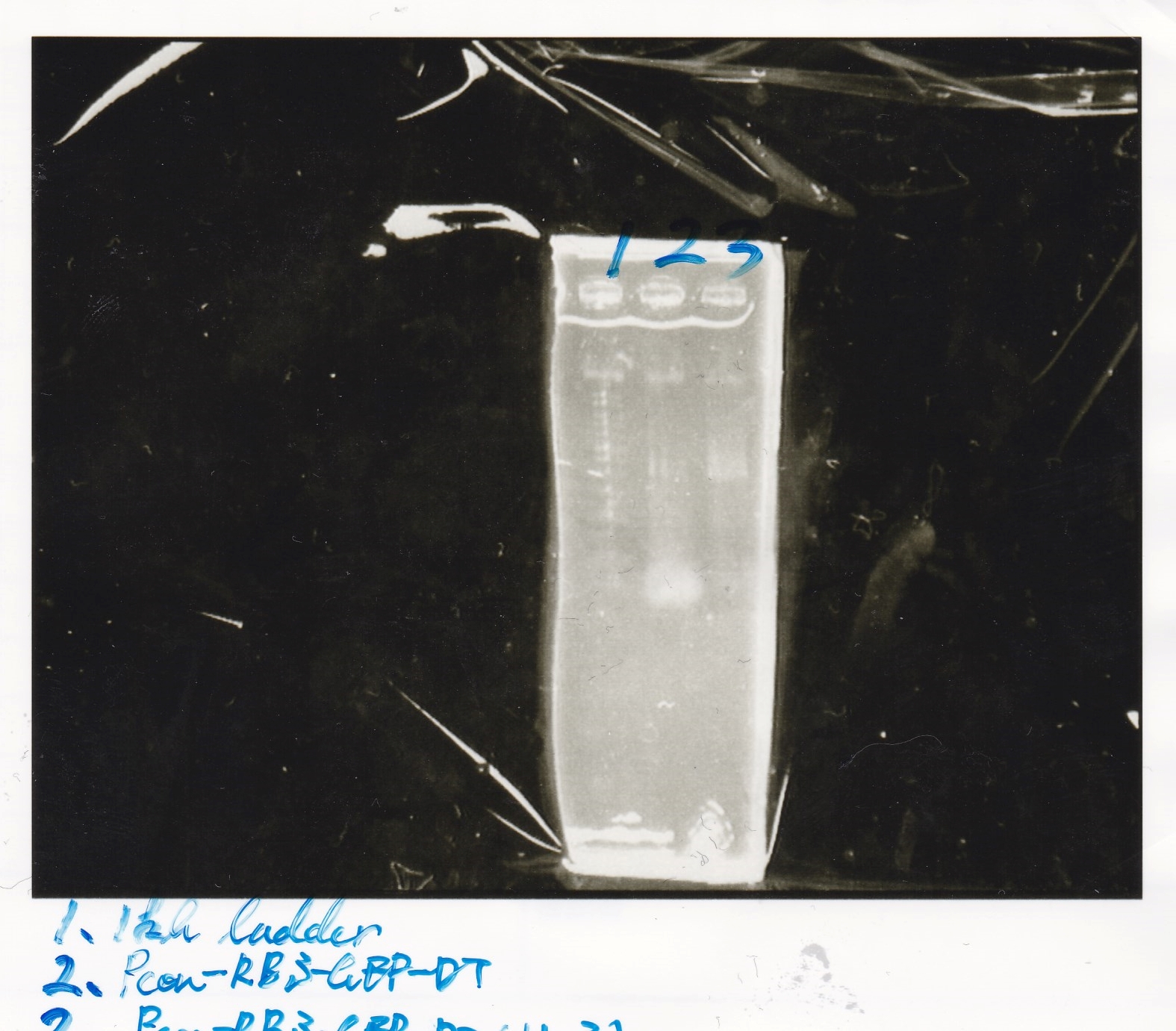
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | -- |
| 2 | pSB1C3 | EcoRI+SpeI |
| 3 | ||
| 4 | ||
| 6 | Pcon-attenuator-aptmer-DT | EcoRI+SpeI |
| 7 | ||
| 8 | ||
| 10 | Pcon-attenuator-aptmer-DT | EcoRI+SpeI |
| 11 | ||
| 12 |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Pcon-attenuator-DT(EcoRI+SpeI) | 6.8 | 1.98 | 0.43 |
| pT181-attenuator(EcoRI+SpeI) | 7.5 | 1.84 | 0.06 |
| pSB1C3(EcoRI+SpeI) | 17.7 | 1.97 | 0.71 |
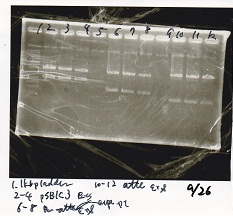
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | -- |
| 3 | pSB4K5 | EcoRI+PstI |
| 4 | pSB4K5 | EcoRI+PstI |
| 5 | pSB4K5 | EcoRI+PstI |
| 7 | pSB6A1 | EcoRI+PstI |
| 8 | pSB6A1 | EcoRI+PstI |
| 9 | pSB6A1 | EcoRI+PstI |
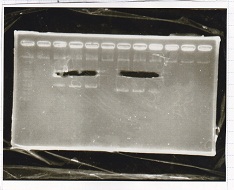
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | pSB4K5 | EcoRI | PstI |
| 2 | pSB4K5 | - | - |
| 3 | 1kbp ladder | - | - |
| 4 | Pcon-attenuator | EcoRI | SpeI |
| 5 | Pcon-attenuator | - | - |
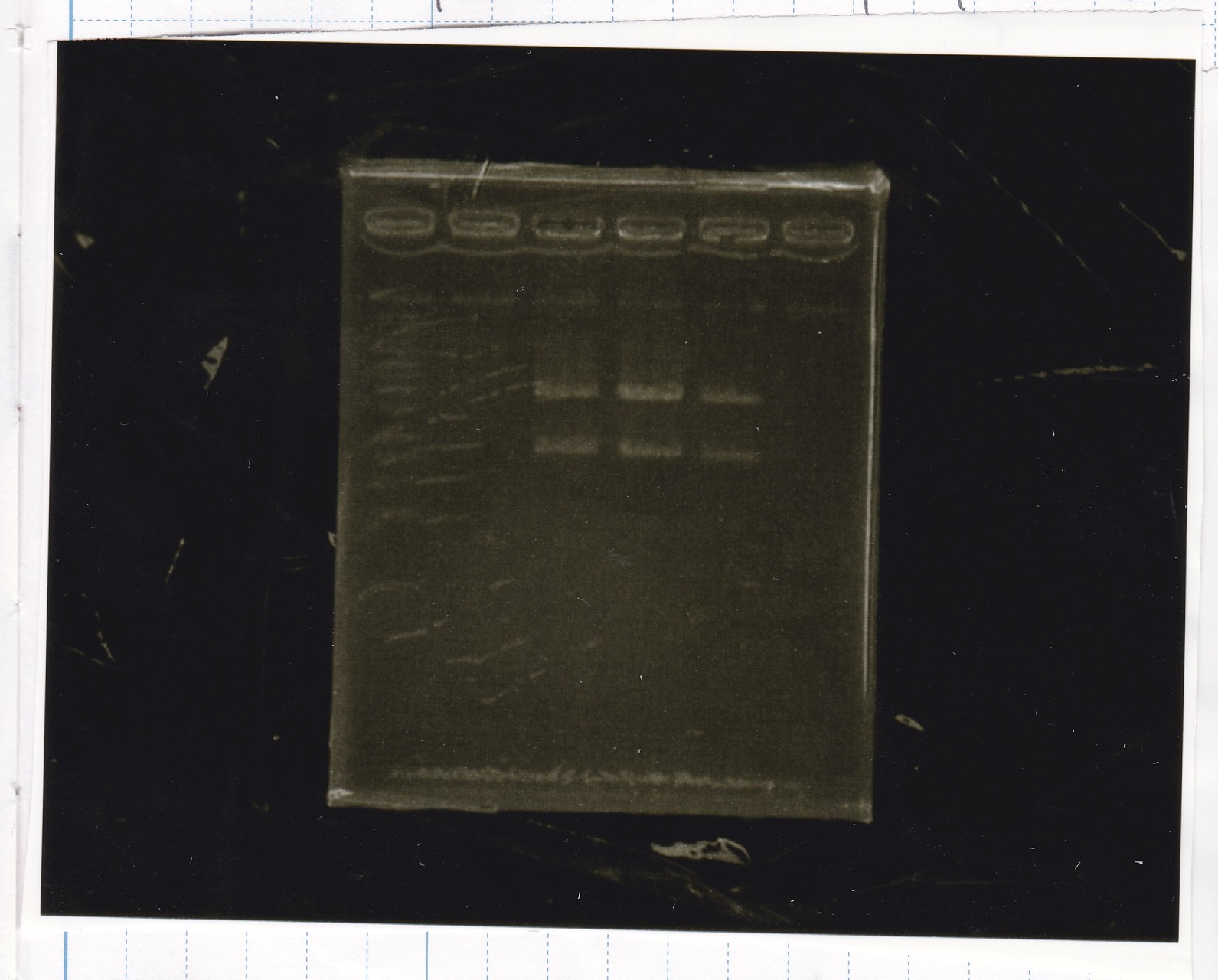
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | RBS-GFP-DT | XbaI | PstI |
| 2 | RBS-GFP-DT | - | - |
| 3 | RBS-GFP-DT | EcoRI | XbaI |
| 4 | 1kbp ladder | - | - |
| 5 | Ptet | EcoRI | SpeI |
| 6 | Ptet | - | - |
Liquid Culture
| Sample | medium |
|---|---|
| Pcon-GFP | Amp |
| RBS-GFP-DT | CP |
| Pcon-attenuator | Amp |
| Pcon-attenuator-aptamer-DT | Amp |
| Pcon-tetR-DT | Amp |
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | 9/17 pSB1C3 (EcoRI+SpeI) 10.0ng/µL | 10µL | 9/26 pT181 attenuator(EcoRI+SpeI) 7.5ng/µL | 1.3µL | 3.5 µL |
| experiment | 9/25 DT (EcoRI+XbaI) 42.8ng/µL | 2.3µL | 9/22 Pcon-pT181 antisense(EcoRI+SpeI) 46.7ng/µL | 1.5µL | 1.9 µL |
| experiment | 9/27 pSB4K5(EcoRI+PstI) 12.9ng/µL | 7.8µL | 9/10 Pcon-attenuator(EcoRI+SpeI) 13.8ng/µL | 8.0µL | 3.5 µL |
| experiment | 9/27 pSB4K5(EcoRI+PstI) 12.9ng/µL | 7.8µL | 9/25 RBS-GFP-DT(XbaI+PstI) 13.9ng/µL | 12µL | 3.5 µL |
| experiment | 9/8 pSB4K5 (EcoRI+SpeI) 17.6ng/µL | 5.7 µL | 8/21 Pcon-GFP-DT(EcoRI+SpeI) 11ng/µL | 12.6µL | 3.5 µL |
Liquid Culture
| Sample | medium |
|---|---|
| Pcon-spinach-DT | plusgrow Amp |
| Pcon-tetRaptamer-DT | plusgrow Amp |
| Pcon-antisense-spinach-DT | plusgrow Amp |
| spinach-DT | plusgrow CP |
RNA Extraction
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 9/26 Pcon-GFP-DT | 423.0 | 1.83 | 1.46 |
| 9/26 Pcon-GFP-DT K | 298.7 | 1.82 | 1.50 |
Electrophoresis
Miniprep
| DNA |
|---|
| Pcon-aptamer-DT |
| Pcon-spinach-DT |
| Pcon-antisense |
| Pcon-tetR-DT |
| Pcon-attenuator-DT |
| Pcon-antisense-spinach-DT |
Transformation
| Name1 | Name2 | Sample1(µL) | Sample2(µL) | Competent Cells(µL) | Total(µL) |
|---|---|---|---|---|---|
| Pcon-attenuator+RBS-GFP-DT(4K5) | Pcon-attenuator-DT(1A2) | 2 | 1 | 30 | 33 |
| Pcon-attenuator+RBS-GFP-DT(4K5) | Pcon-antisense-spinach-DT(1A2) | 2 | 1 | 30 | 33 |
| Pcon-attenuator+RBS-GFP-DT(4K5) | Pcon-tetR aptamer-DT(1A2) | 2 | 1 | 30 | 33 |
| Pcon-RBS-GFP-DT(4K5) | Pcon-attenuator-DT(1A2) | 2 | 1 | 30 | 33 |
| Pcon-RBS-GFP-DT(4K5) | Pcon-antisense-spinach-DT(1A2) | 2 | 1 | 30 | 33 |
| Pcon-RBS-GFP-DT(4K5) | Pcon-tetR aptamer-DT(1A2) | 2 | 1 | 30 | 33 |
| Pcon-attenuator+RBS-GFP-DT(4K5) | -- | 2 | -- | 20 | 22 |
| Pcon-RBS-GFP-DT(4K5) | -- | 2 | -- | 20 | 22 |
| attenuator(1C3) | -- | 2 | -- | 20 | 22 |
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Pcon-RBS-GFP-DT | 137.6 | 1.95 | 1.78 |
| RBS-GFP-DT | 77.0 | 2.06 | 1.96 |
| Pcon-PT181 attenuator | 231.0 | 1.89 | 1.89 |
| Pcon-antisense-Spinach-DT(2) | 130.0 | 1.56 | 1.62 |
| Pcon-aptamer-DT | 218.8 | 1.92 | 1.72 |
| Pcon-attenuator-DT | 180.7 | 1.95 | 1.78 |
| Pcon-Spinach-DT | 177.6 | 1.96 | 1.06 |
| Pcon-antisense-Spinach-DT(1) | 181.1 | 1.96 | 1.92 |
| Pcon-antisense | 152.9 | 2.02 | 1.96 |
| Pcon-RBS+ezR-DT | 164.1 | 1.93 | 1.91 |
Restriction Enzyme Digestion
| 9/27 RBS-GFP-DT | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 12µL | 1µL | 1µL | 3µL | 3µL | 20µL | 40µL |
| NC | 1.3µL | 0µL | 0µL | 1µL | 1µL | 6.7µL | 10µL |
| 9/27 Pcon-tetR-DT | EcoRI | PstI | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|
| 2 cuts | 12.2µL | 1µL | 1µL | 3µL | 12.8µL | 30µL |
| NC | 0.6µL | 0µL | 0µL | 1µL | 8.4µL | 10µL |
RNA Extraction
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 9/27 Pcon-RBS-GFP-DT | 686.6 | 1.99 | 1.34 |
| 9/27 Pcon-RBS-GFP-DT (+High salt) | 127.6 | 1.76 | 0.27 |
DNA sythesis
| genome DNA | DNA volume | genome DNA wipeout buffer | RNase freewater | RT | RT buffer | Primer Mix | total |
|---|---|---|---|---|---|---|---|
| 9/27 Pcon-RBS-GFP-DT | 0.8µL | 1µL | 5.2µL | 0.5µL | 2µL | 0.5µL | 10µL |
| 9/27 pcon-RBS-GFP-DT(+high salt) | 2.5µL | 1µL | 3.5µL | 0.5µL | 2µL | 0.5µL | 10µL |
| 9/27 Pcon-RBS-GFP-DT(revised concentration) | 0.5µL | 1µL | 5.5µL | 0.5µL | 2µL | 0.5µL | 10µL |
Electrophoresis
Liquid Culture
| Sample | medium |
|---|---|
| Pcon spinach DT | plusgrow 2µL(Amp) |
| Pcon aptamer DT | plusgrow 2µL(Amp) |
| Pcon antisense Spinach DT | plusgrow 2µL(Amp) |
| Spinach DT | plusgrow 4µL(CP) |
RT PCR
| genome DNA | KOD plus | 10x buffer for KOD-plus-ver.2 | 2nM dNTPs | 25mM MgSO4 | F primer | R primer | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 15.5 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 49.8°C | 68°C | -- |
| 2min | 10sec | 30sec | 10sec | 30 |
Electrophoresis
| Lane | Sample |
|---|---|
| 1 | Pcon-GFP-DT(GFP) |
| 2 | Pcon-GFP-DT(internal) |
| 3 | Pcon-GFP-DT(HS)(GFP) |
| 4 | Pcon-GFP-DT(internal) |
Digesting cut check
| Lane | Sample |
|---|---|
| 1 | RBS-GFP-DT(XbaI&PstI) |
| 2 | RBS-GFP-DT NC(XbaI&PstI) |
| 3 | 1kbp ladder |
| 4 | Pcon-RBS-tetR-DT(EcoRI&SpeI) |
| 5 | Pcon-RBS-tetR-DT NC(EcoRI&SpeI) |
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | -- |
| 3 | RBS-GFP-DT | Xbal+PstI |
| 4 | RBS-GFP-DT | Xbal+PstI |
| 5 | RBS-GFP-DT | Xbal+PstI |
| 7 | 1kbp ladder | -- |
| 9 | Pcon-tetR-DT | EcoRI+SpeI |
| 10 | Pcon-tetR-DT | EcoRI+SpeI |
| 11 | Pcon-tetR-DT | EcoRI+SpeI |
RNA Extraction
| 0 | 5min | 15min | 20min | 23min | 28min | 33min | 36min | |
|---|---|---|---|---|---|---|---|---|
| aptamer generator | 0.348 | 0.408 | 0.550 | 0.865 | ||||
| Pcon-spinach-DT | 0.250 | 0.240 | 0.305 | 0.426 | 0.471 | 0.554 | 0.668 | 0 |
| GFP generator | 0.389 | 0.420 | 0.516 | 0.884 | ||||
| antisense-spinach | 0.371 | 0.438 | 0.537 | 0.733 | ||||
| Spinach-DT | 0.084 | 0.110 | 0.134 | 0.433 | 0.269 | 0.326 | 0.409 | 0.742 |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Spinach-DT | 83.9 | 1.63 | 0.17 |
| Pcon-tetRaptamer-DT | 425.1 | 2.10 | 0.64 |
| Pcon-RBS-GFP-DT | 262.4 | 2.01 | 0.84 |
| Pcon-antisense-Spinach-DT | 489.1 | 2.05 | 0.68 |
| Pcon-Spinach-DT | 266.6 | 2.01 | 0.66 |
cDNA Synthesis
| 9/27 Spinach-DT | 9/23 Pcon-tetRaptamer-DT | 9/23 Pcon-RBS-GFP-DT | 9/23 Pcon-antisense-spinach-DT | 9/23 Pcon-Spinach-DT | |
|---|---|---|---|---|---|
| RNA | 1 | 1 | 1 | 1 | 1 |
| gDNA wipeout | 6 | 1.2 | 1.9 | 1.0 | 1.9 |
| RNase-free water | 0 | 4.8 | 4.1 | 5.0 | 4.1 |
| RT | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| RT Buffer | 2 | 2 | 2 | 2 | 2 |
| Primer mix | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| total | 10µL | 10µL | 10µL | 10µL | 10µL |
 "
"