Team:DTU-Denmark/Notebook/24 August 2013
From 2013.igem.org
(→Conclusion) |
|||
| (3 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:DTU-Denmark/Templates/StartPage|24 August 2013}} | {{:Team:DTU-Denmark/Templates/StartPage|24 August 2013}} | ||
| - | |||
Navigate to the [[Team:DTU-Denmark/Notebook/23_August_2013|Previous]] or the [[Team:DTU-Denmark/Notebook/25_August_2013|Next]] Entry | Navigate to the [[Team:DTU-Denmark/Notebook/23_August_2013|Previous]] or the [[Team:DTU-Denmark/Notebook/25_August_2013|Next]] Entry | ||
=Lab 208= | =Lab 208= | ||
| Line 6: | Line 5: | ||
==Main purpose== | ==Main purpose== | ||
<hr/> | <hr/> | ||
| + | *fix problem with gels | ||
| + | *run gels | ||
==Who was in the lab== | ==Who was in the lab== | ||
| Line 13: | Line 14: | ||
==Procedure== | ==Procedure== | ||
<hr/> | <hr/> | ||
| + | Running and loading gels. | ||
==Results== | ==Results== | ||
| Line 99: | Line 101: | ||
==Conclusion== | ==Conclusion== | ||
<hr/> | <hr/> | ||
| + | There is no visible difference between the two power-supplies and loading dyes. Probably the TBE we made from powder was not good, we will use TAE from now on. | ||
Navigate to the [[Team:DTU-Denmark/Notebook/23_August_2013|Previous]] or the [[Team:DTU-Denmark/Notebook/25_August_2013|Next]] Entry | Navigate to the [[Team:DTU-Denmark/Notebook/23_August_2013|Previous]] or the [[Team:DTU-Denmark/Notebook/25_August_2013|Next]] Entry | ||
{{:Team:DTU-Denmark/Templates/EndPage}} | {{:Team:DTU-Denmark/Templates/EndPage}} | ||
Latest revision as of 17:58, 28 September 2013
24 August 2013
Contents |
Lab 208
Main purpose
- fix problem with gels
- run gels
Who was in the lab
Kristian, Henrike
Procedure
Running and loading gels.
Results
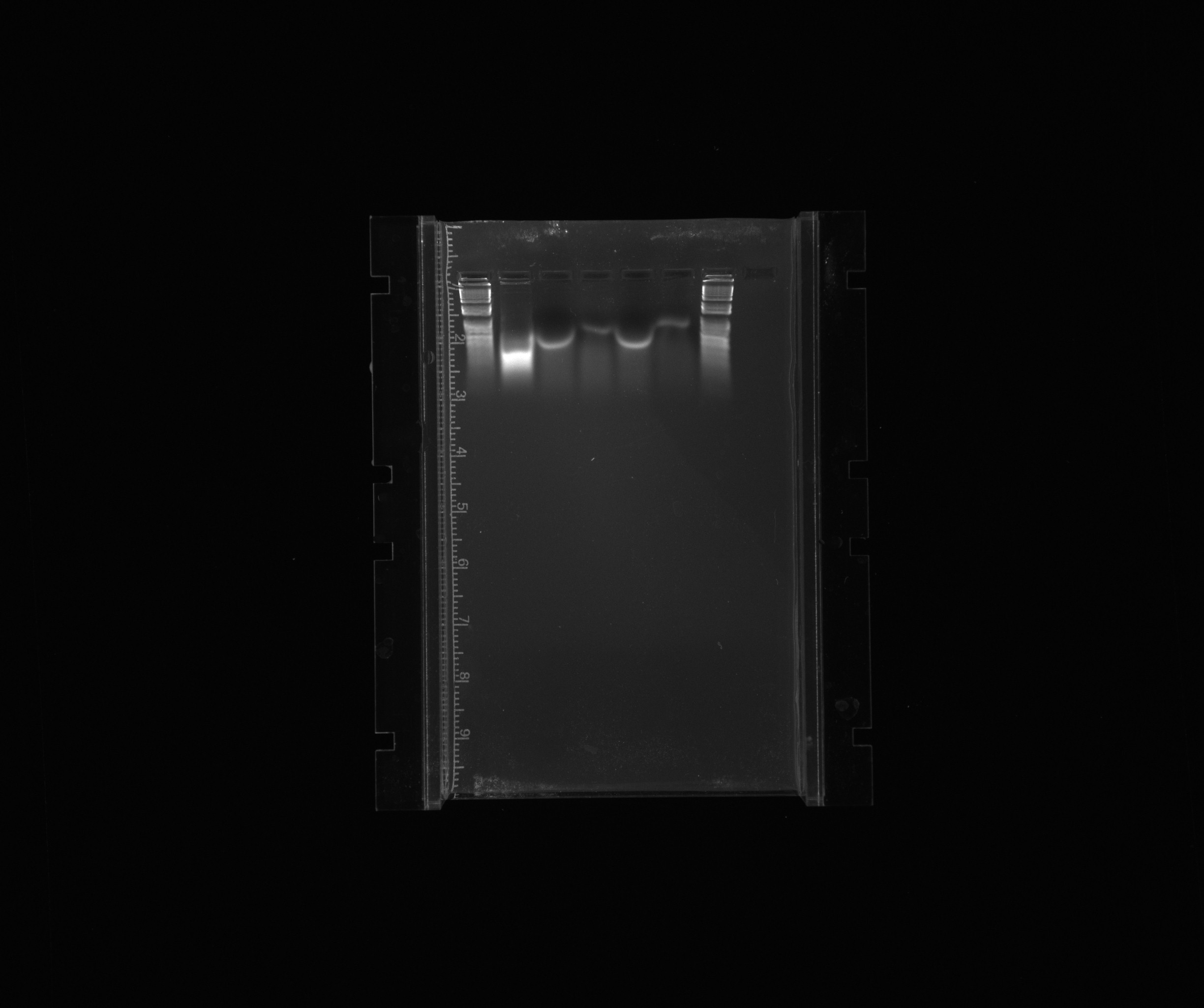
Gels
- 1 kb
- colony PCR NirG
- constitutive reference promoter, GC buffer
- constitutive reference promoter, GC buffer + 3% DMSO
- constitutive reference promoter, HF buffer
- constitutive reference promoter, HF buffer + 3% DMSO
- 1 kb
Gel could not be read and will be redone.
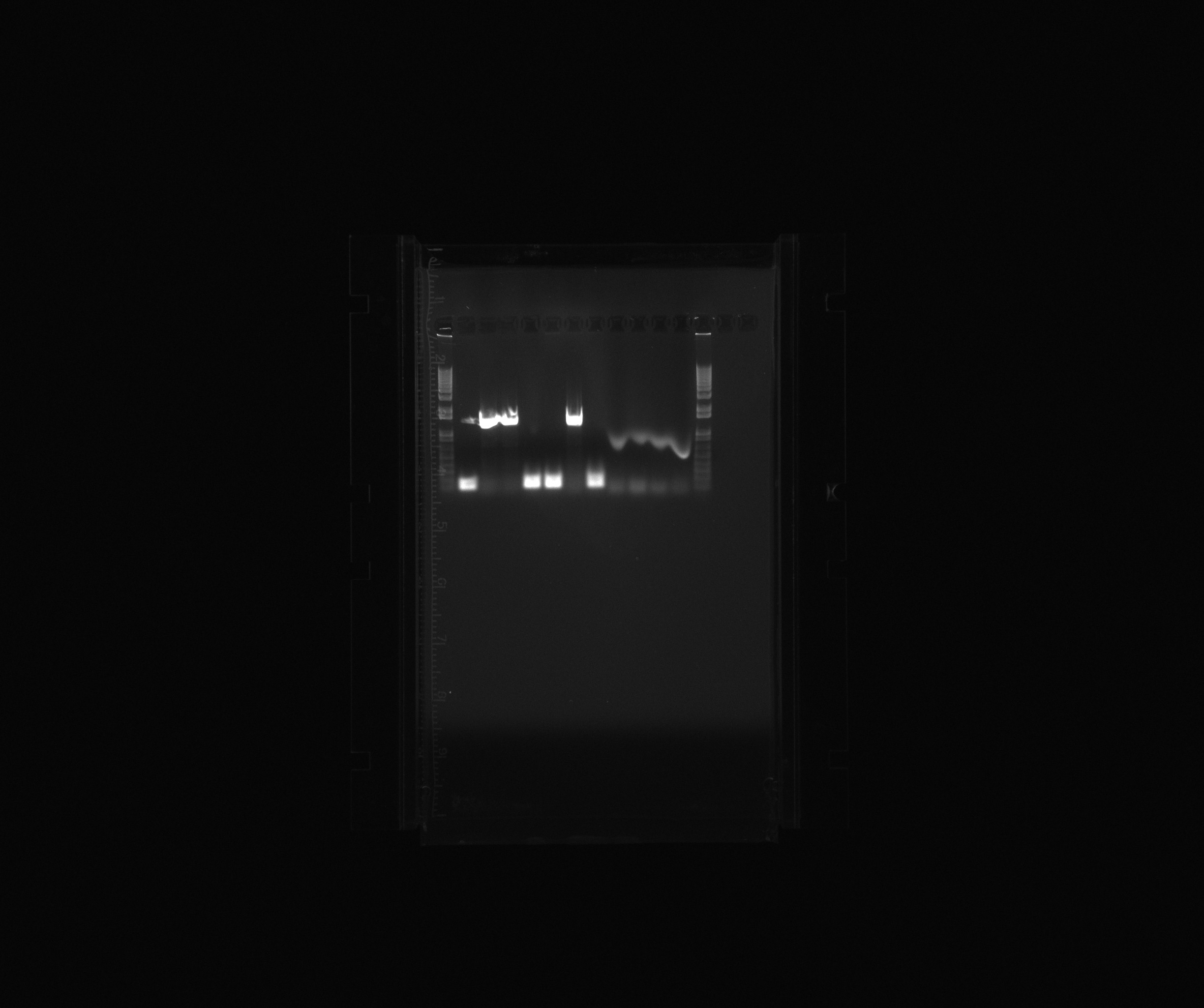
- 1 kb
- colony PCR cycAX, colony 2
- colony PCR cycAX, colony 3
- colony PCR cycAX, colony 4
- colony PCR cycAX, colony 6
- colony PCR cycAX, colony 7
- colony PCR cycAX, colony 8
- colony PCR cycAX, colony 9
- constitutive reference promoter, GC buffer
- constitutive reference promoter, GC buffer + 3% DMSO
- constitutive reference promoter, HF buffer
- constitutive reference promoter, HF buffer + 3% DMSO
- 1 kb
The insert is confirmed for the colonies 3, 4 and 8.
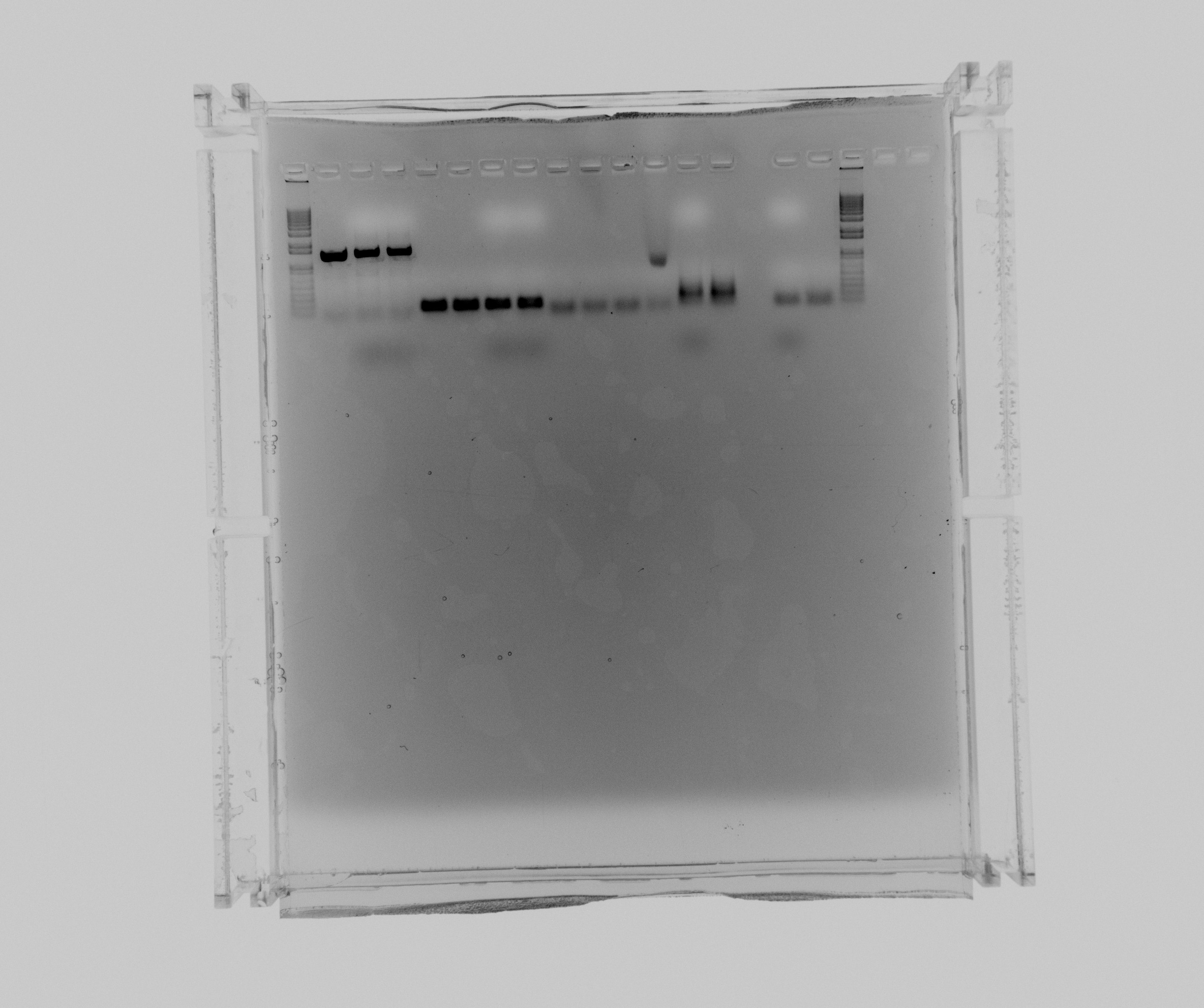
Gel optimization test
Made two gels with TAE buffer to run on the two different power supplies and used two different loading dyes to try and resolve the problem with the bad gels we've been having.
Loading dyes: Agarose Blue was made by us, GelPilot is supplied by Qiagen. The const ref samples were mixed with agarose blue before, so we cannot test the other loading dye on those.
Run on the old power supply (the grey one):
- 1 kb
- cycAX col 3, agarose blue
- cycAX col 4, GelPilot
- cycAX col 8, GelPilot
- cycAX col 2, agarose blue
- cycAX col 6, agarose blue
- cycAX col 7, GelPilot
- cycAX col 9, GelPilot
- constitutive reference promoter, GC buffer
- constitutive reference promoter, GC buffer + 3% DMSO
- constitutive reference promoter, HF buffer
- constitutive reference promoter, HF buffer + 3% DMSO
- NirG col, agarose blue
- NirG col, GelPilot
- HAO col, agarose blue
- HAO col, GelPilot
- 1 kb
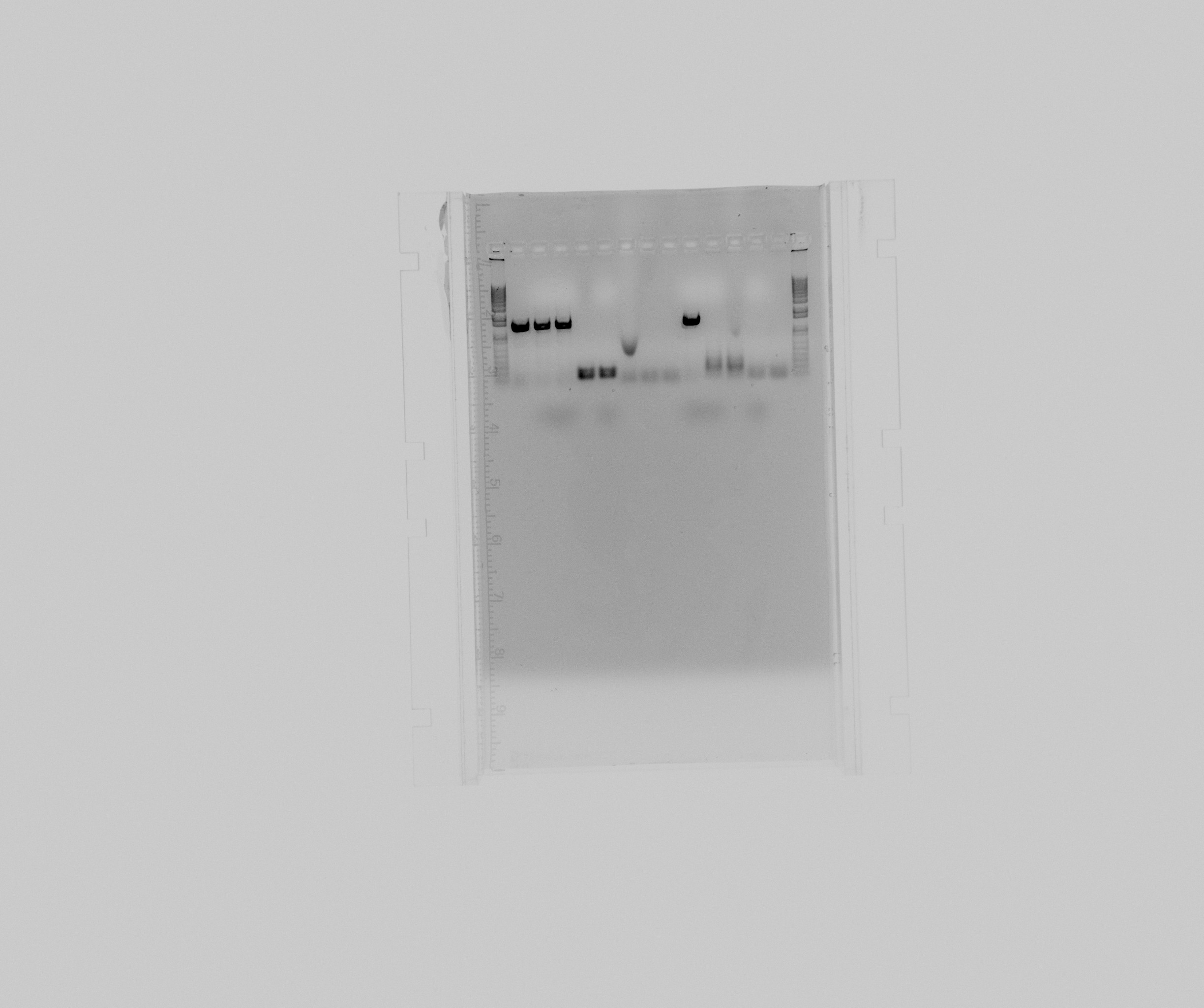
Run on the new power supply (the green one):
- 1 kb
- cycAX col 3, agarose blue
- cycAX col 4, GelPilot
- cycAX col 8, GelPilot
- cycAX col 2, agarose blue
- cycAX col 7, GelPilot
- constitutive reference promoter, GC buffer
- constitutive reference promoter, GC buffer + 3% DMSO
- constitutive reference promoter, HF buffer
- cycAX col 3, GelPilot
- NirG col, agarose blue
- NirG col, GelPilot
- HAO col, agarose blue
- HAO col, GelPilot
- 1 kb
Conclusion
There is no visible difference between the two power-supplies and loading dyes. Probably the TBE we made from powder was not good, we will use TAE from now on.
Navigate to the Previous or the Next Entry
 "
"