Team:Paris Saclay/Notebook/August/9
From 2013.igem.org
(Difference between revisions)
CarolineMir (Talk | contribs) (→1 - Tranduction of Km in MG1655Z1) |
|||
| Line 26: | Line 26: | ||
|} | |} | ||
| - | Protocol : [[Team:Paris_Saclay | + | Protocol : [[Team:Paris_Saclay/transduction|Transduction]] |
Our Mutant bacteria was called BW : Δfnr::Km. | Our Mutant bacteria was called BW : Δfnr::Km. | ||
Revision as of 13:11, 29 September 2013
Notebook : August 9
Lab work
A - Aerobic/Anaerobic regulation system
Objective : characterize Bba_K1155000, Bba_K1155004, Bba_K1155005, Bba_K1155006
1 - Tranduction of Km in MG1655Z1
Abdou, Anaïs, Damir, Nadia, XioaJing
|
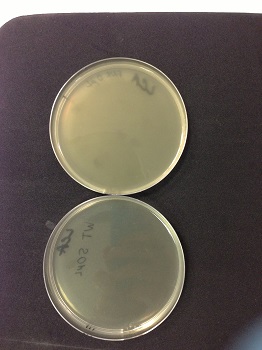
We observed lysis areas. We will continue the transduction protocol. |
|
Picture: lysed cells comparison.
|
Protocol : Transduction
Our Mutant bacteria was called BW : Δfnr::Km. Our wild type bacteria was called MG1655Z1. We used kanamycine antibiotic.
Objective : obtaining Bba_K1155007
1 - Extraction of Bba_K115007 from DH5α
Abdou
Protocol : Hight copy plamid extraction
We used colonies number 10, 14 and 15.
Nanodrop
- Bba_K1155007 in clone 10 : 38ng/µl
- Bba_K1155007 in clone 14 : 48.5ng/µl
- Bba_K1155007 in clone 15 : 52 ng/µl
|
The extraction was good. We will sequence our plasmid. |
A - Aerobic/Anaerobic regulation system / B - PCB sensing system
Objective : Obtaining FNR and BphR2 proteins
1 - Electrophoresis of the PCR of BphR2 Part I, BphR2 Part II, RBS_BphR2 Part I, FNR Part I, FNR Part II, RBS_FNR Part I to check the gel purification
| IMAGE |
|
Expected size :
- BphR2 Part I :
- BphR2 Part II :
- RBS-BphR2 Part I :
- FNR Part I :
- FNR Part II :
- RBS-FNR PartI :
|
We lost all our PCR fragments. We will do the PCR again. |
2 - PCR of BphR2 Part I, BphR2 Part II, RBS-BphR2 Part I, FNR Part I, FNR Part II, RBS-FNR Part I
Anaïs, Damir, Nadia, XiaoJing
Used quantities :
- Bphr2 Part I :
- Oligo 54F : 1µL
- Oligo 55R : 1µL
- Buffer Phusion : 10µL
- DNA of Pseudomonas pseudoalcaligenes : 1µL
- dNTP : 1µL
- Phusion : 0.5µL
- H2O : 35.5µL
- Bphr2 Part II :
- Oligo 56F : 1µL
- Oligo 57R : 1µL
- Buffer Phusion : 10µL
- DNA Pseudomonas pseudoalcaligenes : 1µL
- dNTP : 1µL
- Phusion : 0.5µL
- H2O : 35.5µL
- RBS-Bphr2 Part I :
- Oligo 58F : 1µL
- Oligo 57R : 1µL
- Buffer Phusion : 10µL
- DNA Pseudomonas pseudoalcaligenes : 1µL
- dNTP : 1µL
- Phusion : 0.5µL
- H2O : 35.5µL
- FNR Part I :
- Oligo 59F : 1µL
- Oligo 60R : 1µL
- Buffer Phusion : 10µL
- DNA Escherichia coli : 1µL
- dNTP : 1µL
- Phusion : 0.5µL
- H2O : 35.5µL
- FNR Part II :
- Oligo 61F : 1µL
- Oligo 62R : 1µL
- Buffer Phusion : 10µL
- DNA Escherichia coli : 1µL
- dNTP : 1µL
- Phusion : 0.5µL
- H2O : 35.5µL
- RBS-FNR Part I :
- Oligo 63F : 1µL
- Oligo 62R : 1µL
- Buffer Phusion : 10µL
- DNA Escherichia coli : 1µL
- dNTP : 1µL
- Phusion : 0.5µL
- H2O : 35.5µL
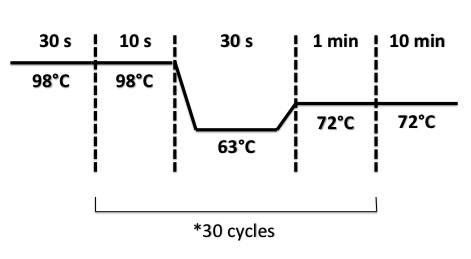
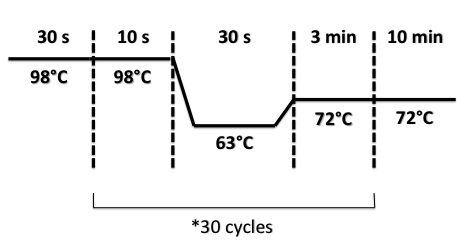
PCR Program :
- BphR2 Part I, BphR2 Part II, RBS-BphR2 Part I :
- FNR Part I, FNR Part II, RBS-FNR Part I :
| Previous day | Back to calendar | Next day |
 "
"