Team:DTU-Denmark/ToxicityExperiment
From 2013.igem.org
(→Discussion) |
(→References) |
||
| Line 62: | Line 62: | ||
<div style="clear: both;"></div> | <div style="clear: both;"></div> | ||
</div> | </div> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{{:Team:DTU-Denmark/Templates/EndPage}} | {{:Team:DTU-Denmark/Templates/EndPage}} | ||
Revision as of 09:33, 4 October 2013
Toxicity Experiment
Contents |
Description
Determine whether the ions nitrite, nitrate and ammonium are toxic to E. coli, and at what concentration. This experiment will use the Biolector to grow an E. coli strain that constitutively expresses RFP in the presence of these ions (at a range of concentrations) to determine the OD and fluorescence of the cells as a proxy for growth.
Results
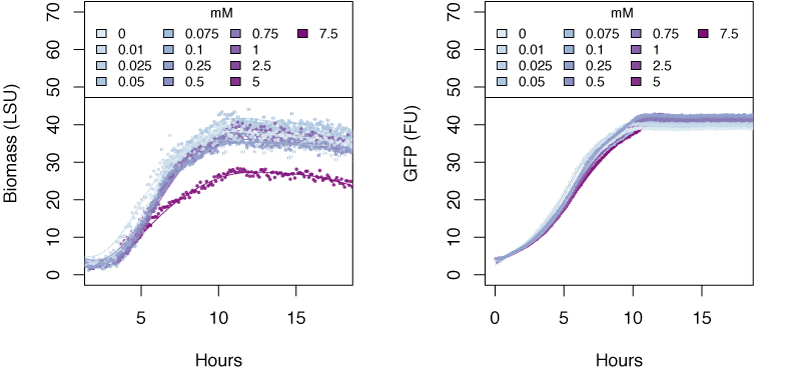
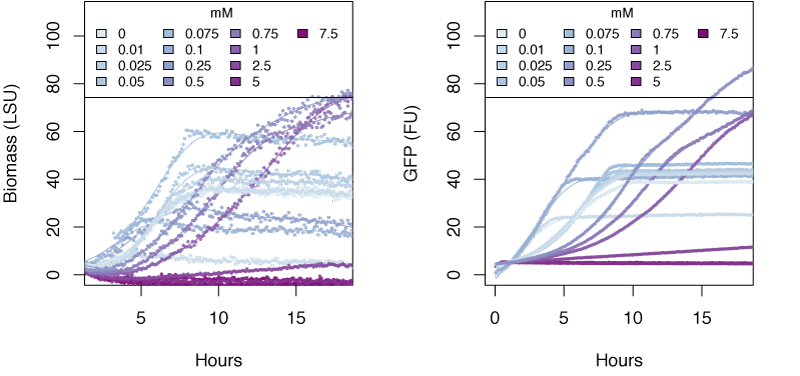
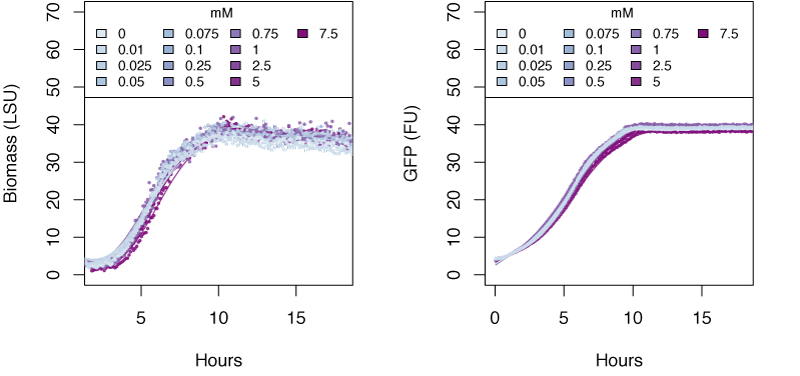
The graphs in the following sections show the response in biomass and fluorescence of GFP over time for varying concentrations of each ion. The data has been baseline adjusted by using a script from Chris Workman (unpublished). The horizontal facets show concentrations in mM.
Ammonium
Nitrate
Nitrite
Conclusions
Very high concentrations of ammonium ≥500 mM were able to inhibit growth of E. coli. Nitrate at concentrations of 25mM and greater also inhibits growth of E. coli, whereas concentrations of nitrate at 5mM and greater show delayed expression of GFP and slower growth. The tested concentrations of nitrite were not sufficient to inhibit growth.
Discussion
The average concentration of ammonium in wastewater treatment plants is 70 mg/L [http://www.gnest.org/journal/Vol1_No1/Sotirakou.pdf], which is much less than 500mM that we found to be toxic to E. coli. If we assume the ammonia is stoichiometrically converted to nitrite via our mutants, the concentrations of nitrite that will be produced with this volume of ammonium will also not be toxic to the cell. We do not expect that a significant amount of nitrate will be produced during this process, since E. coli contains the Nap pathway that converts nitrate to nitrite. Therefore, we can expect that our transformants will survive in this environment.
References
- Mark A. Sutton, Gilles Billen, Albert Bleeker, Jan Willem Erisman, Peringe Grennfelt, Hans van Grinsven, Bruna Grizzetti, Clare M. Howard and Adrian Leip; Technical summary, European Nitrogen Assessment
- Yaniv D. Scherson, George F. Wells, Sung-Geun Woo, Jangho Lee, Joonhong Park, Brian J. Cantwell and Craig S. Criddle; Nitrogen removal with energy recovery through N2O decomposition; Energy & Environmental Science. Issue 1 2013.
- U.S. Energy Information Administration, Frequently Asked Questions: How much electricity does an American home use?
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., and Tanabe, M.; KEGG for integration and interpretation of large-scale molecular datasets. Nucleic Acids Res. 40, D109-D114 (2012).
- Kanehisa, M. and Goto, S.; KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27-30 (2000).
- Zumft, Walter G. (1997) Cell Biology and Molecular Basis of Denitrification. Microbiology and Molecular Biology Reviews, December 1997 p. 533–616
- Ingledew, WJ and Poole, RK (1984) The Respiratory Chains of Escherichia coli. Microbiological Reviews, Sept. 1984, p. 222-271
- Nour-Eldin, HH et al (2010). USER cloning and USER fusion: the ideal cloning techniques for small and big laboratories. Methods Mol Biol. 2010;643:185-200. doi: 10.1007/978-1-60761-723-5_13.
- Nørholm, Morten HH. "A mutant Pfu DNA polymerase designed for advanced uracil-excision DNA engineering." BMC biotechnology 10.1 (2010): 21.
- Frandsen, Rasmus, et al. "Efficient four fragment cloning for the construction of vectors for targeted gene replacement in filamentous fungi." BMC molecular biology 9.1 (2008): 70.
 "
"