Team:Paris Saclay/Notebook/August/30
From 2013.igem.org
(→1 - Results of liquid culture of Pfnr-RBS-Amil CP-Term in pSB1C3 in aerobic and anaerobic conditions) |
(→Notebook : August 30) |
||
| Line 20: | Line 20: | ||
[[File:PsPfnr3008.jpg|600px]] | [[File:PsPfnr3008.jpg|600px]] | ||
| - | In anaerobic condition, the constitutive promoter Pndh* | + | In anaerobic condition, FNR protein (active form) binds to the constitutive promoter Pndh* and repressed ''amilCP'' expression. Therefore, the bacteria have no colour. |
| - | In aerobic condition, | + | In aerobic condition, FNR protein is inactive and can not bind to constitutive promoter Pndh*, ''amilCP'' is expressed and we can see the bacteria have violet colour. |
| - | ===='''2 - Results of culture of Pfnr with RBS- | + | ===='''2 - Results of culture of Pfnr with RBS-AmilCP-Term in pSB1C3, PnirB with RBS-LacZ-Term in pSB1C3 in aerobic and anaerobic conditions'''==== |
XiaoJing | XiaoJing | ||
| Line 30: | Line 30: | ||
{| | {| | ||
| style="border:1px solid black;padding:5px;background-color:#DE;" | | | style="border:1px solid black;padding:5px;background-color:#DE;" | | ||
| - | Purification of 08/29/13 didn't work. We have blue colonies for | + | Purification of 08/29/13 didn't work. We have blue colonies for Pndh* with RBS-AmilCP-Term in pSB1C3 in aerobic and anaerobic conditions. We also have blue colonies for PnirB with RBS-LacZ-Term in pSB1C3 in anaerobic conditions. |
|} | |} | ||
| Line 36: | Line 36: | ||
[[File:PsNirBcult3008.jpg|500px]] | [[File:PsNirBcult3008.jpg|500px]] | ||
| - | ===='''3 - Ligation of | + | ===='''3 - Ligation of Pndh* with RBS-LacZ-Term in pSB1C3 '''==== |
XiaoJing | XiaoJing | ||
Used quantities : | Used quantities : | ||
| - | * | + | * Pndh* : 8µL |
* RBS-LacZ-Term in pSB1C3 : 3µL | * RBS-LacZ-Term in pSB1C3 : 3µL | ||
* Buffer ligation : 2µL | * Buffer ligation : 2µL | ||
| Line 47: | Line 47: | ||
* H2O : 6µL | * H2O : 6µL | ||
| - | ===='''4 - Transformation of ligation of | + | ===='''4 - Transformation of ligation of Pndh* with RBS-LacZ-Term in pSB1C3 in DH5α strain '''==== |
XiaoJing | XiaoJing | ||
| Line 53: | Line 53: | ||
Protocol : [[Team:Paris_Saclay/Protocols/Transformation|Bacterial transformation]] | Protocol : [[Team:Paris_Saclay/Protocols/Transformation|Bacterial transformation]] | ||
| - | We incubate | + | We incubate the bacteria in LB-chloramphenicol-Xgal plate at 37°C in aerobic conditions. |
| - | ===='''5 - Ligation of Pfnr with RBS-LacZ-Term in pSB3K3 and | + | ===='''5 - Ligation of Pfnr with RBS-LacZ-Term in pSB3K3 and PnarK with RBS-LacZ-Term in pSB3K3 '''==== |
XiaoJing | XiaoJing | ||
Used quantities : | Used quantities : | ||
| - | * | + | * Pndh*, PnarK : 3µL |
* RBS-LacZ-Term : 3µL | * RBS-LacZ-Term : 3µL | ||
* pSB3K3 : 5µL | * pSB3K3 : 5µL | ||
| Line 67: | Line 67: | ||
* H2O : 6µL | * H2O : 6µL | ||
| - | ===='''6 - Transformation of ligation of Pfnr with RBS-LacZ-Term in | + | ===='''6 - Transformation of ligation of Pfnr with RBS-LacZ-Term in pSB3K3 and PnarK with RBS-LacZ-Term in pSB3K3 in DH5α strain '''==== |
XiaoJing | XiaoJing | ||
| Line 73: | Line 73: | ||
Protocol : [[Team:Paris_Saclay/Protocols/Transformation|Bacterial transformation]] | Protocol : [[Team:Paris_Saclay/Protocols/Transformation|Bacterial transformation]] | ||
| - | We incubate | + | We incubate the bacteria at 37°C with LB-Kanamycine-Xgal in anaerobic conditions for PnarK with RBS-LacZ-Term in PSB3K3. |
| - | ===='''7 - Electrophoresis of | + | We incubate the bacteria at 37°C with LB-Kanamycine-Xgal in aerobic conditions for Pndh* with RBS-LacZ-Term in pSB3K3. |
| + | |||
| + | ===='''7 - Electrophoresis of PCR Colony of Pfnr with RBS_AmilCP-Term in DH5α strain '''==== | ||
XiaoJing | XiaoJing | ||
| Line 83: | Line 85: | ||
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
* Well 1 : 6µL DNA Ladder | * Well 1 : 6µL DNA Ladder | ||
| - | * Well 2 : 20µL of Pfnr with RBS_AmilCP-Term clone 1+4µL of 6X loading dye | + | * Well 2 : 20µL of Pfnr with RBS_AmilCP-Term clone 1 + 4µL of 6X loading dye |
| - | * Well 3 : 20µL of Pfnr with RBS_AmilCP-Term clone 2+4µL of 6X loading dye | + | * Well 3 : 20µL of Pfnr with RBS_AmilCP-Term clone 2 + 4µL of 6X loading dye |
| - | * Well 4 : 20µL of Pfnr with RBS_AmilCP-Term clone 3+4µL of 6X loading dye | + | * Well 4 : 20µL of Pfnr with RBS_AmilCP-Term clone 3 + 4µL of 6X loading dye |
| - | * Well 5 : 20µL of Pfnr with RBS_AmilCP-Term clone 4+4µL of 6X loading dye | + | * Well 5 : 20µL of Pfnr with RBS_AmilCP-Term clone 4 + 4µL of 6X loading dye |
* Gel : 1.0% | * Gel : 1.0% | ||
|} | |} | ||
| Line 102: | Line 104: | ||
===='''Objective : obtaining FNR and BphR2 proteins'''==== | ===='''Objective : obtaining FNR and BphR2 proteins'''==== | ||
| - | ===='''1 - Electrophoresis of | + | ===='''1 - Electrophoresis of PCR Colony of FNR, RBS-FNR and RBS-BphR2'''==== |
{| | {| | ||
| Line 108: | Line 110: | ||
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
* Well 1 : 6µL DNA Ladder | * Well 1 : 6µL DNA Ladder | ||
| - | * Well 2 to 17 : 5µL of FNR+1µl of 6X loading dye | + | * Well 2 to 17 : 5µL of FNR + 1µl of 6X loading dye |
| - | * Well 18 to 26 : 5µL of RBS-BphR2+1µl of 6X loading dye | + | * Well 18 to 26 : 5µL of RBS-BphR2 + 1µl of 6X loading dye |
* Well 27 : 6µL DNA Ladder | * Well 27 : 6µL DNA Ladder | ||
| - | * Well 28 to 41 : 5µL of RBS-FNR+1µl of 6X loading dye | + | * Well 28 to 41 : 5µL of RBS-FNR + 1µl of 6X loading dye |
| - | * Well 42 to 49 : 5µL of RBS-BphR2+1µl of 6X loading dye | + | * Well 42 to 49 : 5µL of RBS-BphR2 + 1µl of 6X loading dye |
* WEll 50 : 6µL DNA Ladder | * WEll 50 : 6µL DNA Ladder | ||
* Gel : 1% | * Gel : 1% | ||
Revision as of 17:37, 4 October 2013
Notebook : August 30
Lab work
A - Aerobic/Anaerobic regulation system
Objective : characterize BBa_K1155000, BBa_K1155004, BBa_K1155005, BBa_K1155006
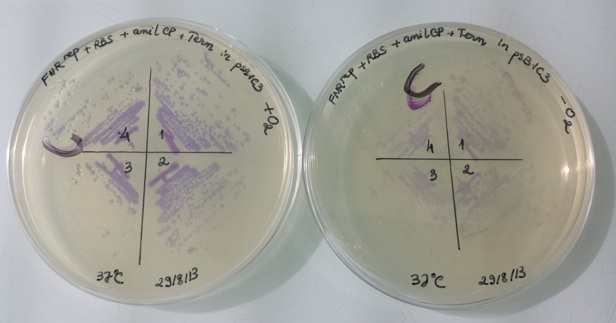
1 - Results of liquid culture of Pfnr-RBS-Amil CP-Term in pSB1C3 in aerobic and anaerobic conditions
XiaoJing
|
IT WORKS !!! |
In anaerobic condition, FNR protein (active form) binds to the constitutive promoter Pndh* and repressed amilCP expression. Therefore, the bacteria have no colour.
In aerobic condition, FNR protein is inactive and can not bind to constitutive promoter Pndh*, amilCP is expressed and we can see the bacteria have violet colour.
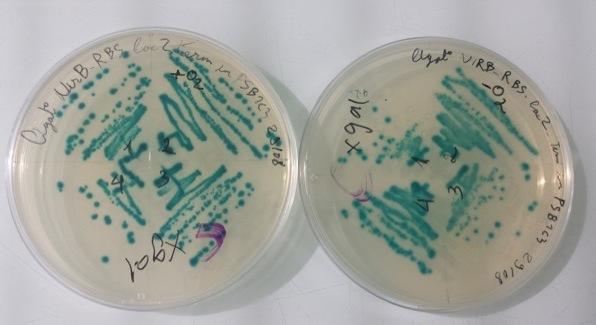
2 - Results of culture of Pfnr with RBS-AmilCP-Term in pSB1C3, PnirB with RBS-LacZ-Term in pSB1C3 in aerobic and anaerobic conditions
XiaoJing
|
Purification of 08/29/13 didn't work. We have blue colonies for Pndh* with RBS-AmilCP-Term in pSB1C3 in aerobic and anaerobic conditions. We also have blue colonies for PnirB with RBS-LacZ-Term in pSB1C3 in anaerobic conditions. |
3 - Ligation of Pndh* with RBS-LacZ-Term in pSB1C3
XiaoJing
Used quantities :
- Pndh* : 8µL
- RBS-LacZ-Term in pSB1C3 : 3µL
- Buffer ligation : 2µL
- Ligase : 1µL
- H2O : 6µL
4 - Transformation of ligation of Pndh* with RBS-LacZ-Term in pSB1C3 in DH5α strain
XiaoJing
Protocol : Bacterial transformation
We incubate the bacteria in LB-chloramphenicol-Xgal plate at 37°C in aerobic conditions.
5 - Ligation of Pfnr with RBS-LacZ-Term in pSB3K3 and PnarK with RBS-LacZ-Term in pSB3K3
XiaoJing
Used quantities :
- Pndh*, PnarK : 3µL
- RBS-LacZ-Term : 3µL
- pSB3K3 : 5µL
- Buffer ligase : 2µL
- Ligase : 1µL
- H2O : 6µL
6 - Transformation of ligation of Pfnr with RBS-LacZ-Term in pSB3K3 and PnarK with RBS-LacZ-Term in pSB3K3 in DH5α strain
XiaoJing
Protocol : Bacterial transformation
We incubate the bacteria at 37°C with LB-Kanamycine-Xgal in anaerobic conditions for PnarK with RBS-LacZ-Term in PSB3K3.
We incubate the bacteria at 37°C with LB-Kanamycine-Xgal in aerobic conditions for Pndh* with RBS-LacZ-Term in pSB3K3.
7 - Electrophoresis of PCR Colony of Pfnr with RBS_AmilCP-Term in DH5α strain
XiaoJing
| [[]] |
|
Expected sizes :
- Pfnr with RBS_AmilCP-Term : 1000bp
|
We obtain fragments at the right size. |
A - Aerobic/Anaerobic regulation system / B - PCB sensor system
Objective : obtaining FNR and BphR2 proteins
1 - Electrophoresis of PCR Colony of FNR, RBS-FNR and RBS-BphR2
| ]] |
|
Expect sizes :
- FNR : 1096 bp
- RBS-FNR : 1014 bp
- RBS-BphR2 : 1469 bp
|
We obtain fragment at right size for FNR and RBS-FNR. The Gisbon assembly of 08/26/13 was good. |
| Previous day | Back to calendar | Next day |
 "
"