Team:Heidelberg/Templates/DelH week13
From 2013.igem.org
(→Electroporation) |
(→Result) |
||
| (5 intermediate revisions not shown) | |||
| Line 31: | Line 31: | ||
* Of each miniprep, 3 µl were loaded | * Of each miniprep, 3 µl were loaded | ||
[[File:Heidelberg_20130724 miniprepBB.png|200px|thumb|right|'''Fig.13.1''' Analytical gel for checking concentration of purified BB (PSB6A1-lacI-mRFP) (loaded 3 µL of PCR) <br>''l1'' 2log ladder ''l2:'' pSB6A1-lacI-mRFP <br> bands at 5 Kb = is ok, because BB is not linear]] | [[File:Heidelberg_20130724 miniprepBB.png|200px|thumb|right|'''Fig.13.1''' Analytical gel for checking concentration of purified BB (PSB6A1-lacI-mRFP) (loaded 3 µL of PCR) <br>''l1'' 2log ladder ''l2:'' pSB6A1-lacI-mRFP <br> bands at 5 Kb = is ok, because BB is not linear]] | ||
| - | + | ||
At 3Kb and at 8 Kb, there are bands visible. | At 3Kb and at 8 Kb, there are bands visible. | ||
:=> Minipreps are fine. | :=> Minipreps are fine. | ||
<br/> | <br/> | ||
| + | <div style="clear:both"></div> | ||
====PCR Conditions BB.W13.A==== | ====PCR Conditions BB.W13.A==== | ||
{| class="wikitable" style="float:left; margin-right:1em" | {| class="wikitable" style="float:left; margin-right:1em" | ||
| Line 78: | Line 79: | ||
Expected band: 5 Kb | Expected band: 5 Kb | ||
<br/> | <br/> | ||
| - | [[File:Heidelberg_20130725 PCRofBB.png|200px|thumb|right|'''Fig.13. | + | [[File:Heidelberg_20130725 PCRofBB cut.png|200px|thumb|right|'''Fig.13.3''' gel of BB(PSB6A1-lacI-mRFP) (loaded 3 µL of PCR) <br>''l1:'' BB amplified with HM11 & HM12, ''l2:'' BB amplified with HM11 & HM12,''l3:'' 2log ladder <br> specific bands at 5 Kb on both lanes = was cut out]] |
| - | + | <div class="tright" style="clear:none">[[File:Heidelberg_20130725 PCRofBB.png|200px|thumb|right|'''Fig.13.2''' gel of BB(PSB6A1-lacI-mRFP) (loaded 3 µL of PCR) <br>''l1:'' BB amplified with HM11 & HM12, ''l2:'' BB amplified with HM11 & HM12,''l3:'' 2log ladder <br> specific bands at 5 Kb on both lanes = BB is ok]]</div> | |
| + | |||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
<br/> | <br/> | ||
| Line 89: | Line 91: | ||
====Analysis of G0, G1 & G2b on Gel==== | ====Analysis of G0, G1 & G2b on Gel==== | ||
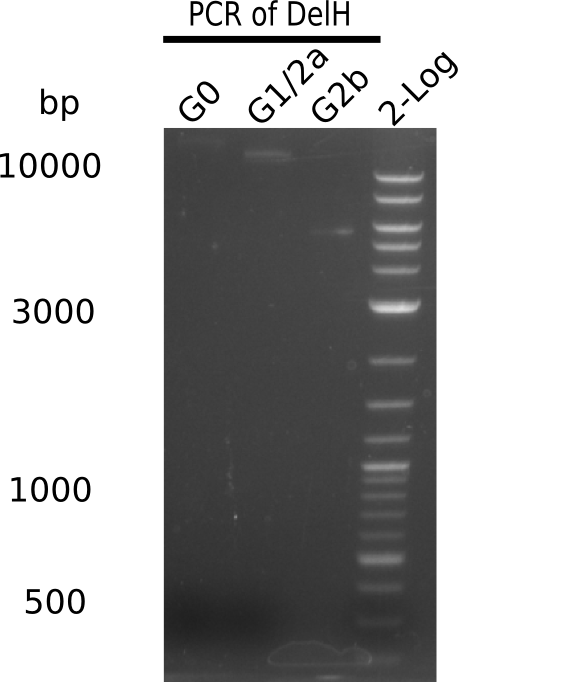
[[File:Heidelberg_20130725 G0 G1 G2b 2log.png|200px|thumb|right|'''Fig.13.4''' analyse-gel for checking concentration of DelH fragments (loaded 3 µL of PCR) <br>''l1'' 1Kb+ ladder ''l2:'' G0 complete DelH-fragment (DN11 & HM08), ''l3'' G1/2a DelH-fragment (DN11 & HM06), ''l4:'' G2b DelH-fragment (DHM07 & HM08) <br> all bands are as expected = result shown in table below]] | [[File:Heidelberg_20130725 G0 G1 G2b 2log.png|200px|thumb|right|'''Fig.13.4''' analyse-gel for checking concentration of DelH fragments (loaded 3 µL of PCR) <br>''l1'' 1Kb+ ladder ''l2:'' G0 complete DelH-fragment (DN11 & HM08), ''l3'' G1/2a DelH-fragment (DN11 & HM06), ''l4:'' G2b DelH-fragment (DHM07 & HM08) <br> all bands are as expected = result shown in table below]] | ||
| - | + | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| Line 101: | Line 103: | ||
|} | |} | ||
<br/> | <br/> | ||
| + | <div style="clear:both"></div> | ||
====Overview on Fragments==== | ====Overview on Fragments==== | ||
| Line 193: | Line 196: | ||
<br/> | <br/> | ||
[[File:Heidelberg_20130728 2log BB G0.png|200px|thumb|right|'''Fig.13.5''' Analytical gel of backbone (pSB6A1-lacI-mRFP) and complete DelH fragment = G0 (loaded 5 µL of PCR) <br> ''l1:''2log ladder, ''l2:''BB,''l3:'' G0 <br> BB and DelH show the expected band at 18 Kb and 4.4 Kb]] | [[File:Heidelberg_20130728 2log BB G0.png|200px|thumb|right|'''Fig.13.5''' Analytical gel of backbone (pSB6A1-lacI-mRFP) and complete DelH fragment = G0 (loaded 5 µL of PCR) <br> ''l1:''2log ladder, ''l2:''BB,''l3:'' G0 <br> BB and DelH show the expected band at 18 Kb and 4.4 Kb]] | ||
| - | + | ||
No product visible of correct size. There is a light band at ~1.5 Kb. | No product visible of correct size. There is a light band at ~1.5 Kb. | ||
:=> Most probably too little DNA loaded. | :=> Most probably too little DNA loaded. | ||
<br/> | <br/> | ||
| + | <div style="clear:both"></div> | ||
====Electroporation==== | ====Electroporation==== | ||
| - | * Electroporation into freshly prepared electrocompetent cell following [ | + | * Electroporation into freshly prepared electrocompetent cell following [https://2013.igem.org/Team:Heidelberg/Project/Methods the protocol] |
* Streaked on LB Amp plates and incubated ON at 37°C | * Streaked on LB Amp plates and incubated ON at 37°C | ||
<br/> | <br/> | ||
| + | |||
====Result==== | ====Result==== | ||
There were few colonies on the plates. Red colonies were picked and screenied by colony-PCR, but none of them was positive. The Gibson assembly has to be repeated. | There were few colonies on the plates. Red colonies were picked and screenied by colony-PCR, but none of them was positive. The Gibson assembly has to be repeated. | ||
<br/> | <br/> | ||
====Preparation of Electrocompetent ''E.coli'' DH10ß==== | ====Preparation of Electrocompetent ''E.coli'' DH10ß==== | ||
| - | According to [ | + | According to [https://2013.igem.org/Team:Heidelberg/Project/Methods the protocol] |
<br/> | <br/> | ||
<br/> | <br/> | ||
| + | |||
===Amplification of Backbone pSB6A1-lacZ-mRFP=== | ===Amplification of Backbone pSB6A1-lacZ-mRFP=== | ||
====Test Restriction Digest==== | ====Test Restriction Digest==== | ||
| Line 222: | Line 228: | ||
<br/> | <br/> | ||
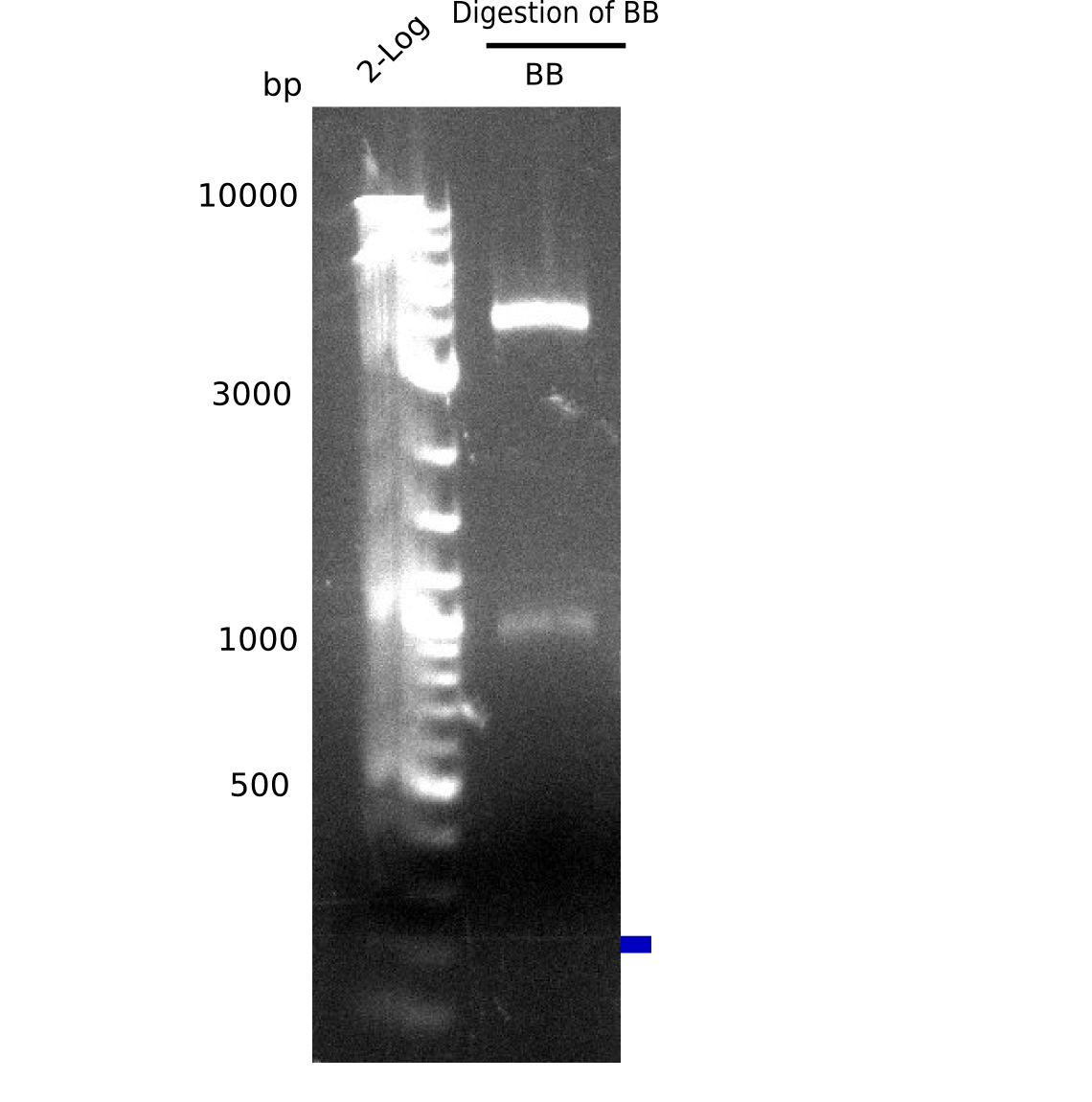
[[File:Heidelberg_20130728 M testdigestofBB.png|200px|thumb|right|'''Fig.13.6'''test digest of Backbone (pSB6A1-lacZ-mRFP) (loaded 20 µL of PCR) <br> ''l1:''2log ladder, ''l2:''BB digested with NotI <br> digested BB show expected band at 4 Kb and 1 Kb => The backbone is ok.]] | [[File:Heidelberg_20130728 M testdigestofBB.png|200px|thumb|right|'''Fig.13.6'''test digest of Backbone (pSB6A1-lacZ-mRFP) (loaded 20 µL of PCR) <br> ''l1:''2log ladder, ''l2:''BB digested with NotI <br> digested BB show expected band at 4 Kb and 1 Kb => The backbone is ok.]] | ||
| - | + | ||
Gel shows shows expeted bands at 4 Kb and 1 Kb. | Gel shows shows expeted bands at 4 Kb and 1 Kb. | ||
:=> Backbone is fine. | :=> Backbone is fine. | ||
<br/> | <br/> | ||
| + | <div style="clear:both"></div> | ||
Latest revision as of 11:00, 25 October 2013
Contents |
22-07 - 28-07-13
Amplification of Backbone pSB6A1-lacZ-mRFP
Miniprep
- 4 colonies were picked and inoculated in 4x 3 ml LB Amp
- Incubated ON at 37°C
- 4x 3 ml were distibuted in 5x 2 ml eppis and centrifuged
- Supernatant was discarded, cell pellet was used for miniprep
- Two minipreps were pooled into 1 column and 180ipreps on another one (every eluation was performed with 50 µl)
Result
- 5 µl were loaded on a gel
- => Gel was not succesful
- => Miniprep didn't work and has to be repeated
Glycerol Stock
Glycerol stocks were prepared from liquid culture not used for miniprep.
Repetiotion of Miniprep
4x 2 ml of Top10 pSB6A1-lacZ-mRFP were minipreped with the Qiagen kit.
Result
| Sample | Performed | Concentration (Nanovue) | P2 Buffer |
|---|---|---|---|
| pSB6A1-lacI-mRFP (1) | upstairs | 88 ng/µl | from upstairs |
| pSB6A1-lacI-mRFP (2) | iGEM lab | 62 ng/µl | new |
| pSB6A1-lacI-mRFP (3) | iGEM lab | 104 ng/µl | new |
| pSB6A1-lacI-mRFP (4) | iGEM lab | 60 ng/µl | old |
- Of each miniprep, 3 µl were loaded
At 3Kb and at 8 Kb, there are bands visible.
- => Minipreps are fine.
PCR Conditions BB.W13.A
| Reagent | Backbone | Backbone |
|---|---|---|
| Template | Miniprep A | Miniprep A |
| Primer fw 10 µM | HM11 | HM11 |
| Primer rev 10 µM | HM12 | HM12 |
| Phusion Flash Ready Mix | 10 µl | 10 µl |
| ddH2O | 7 µl | 7 µl |
| DMSO | - | - |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 (touchdown - 0,5) | 5 | |
| 72 | 1:15 min | |
| 18 | 98 | 1 |
| 67 | 5 | |
| 72 | 1:15 min | |
| 1 | 72 | 5 min |
| 1 | 4 | inf |
Result
Expected band: 5 Kb
Specific expected band at 5 Kb
- => Fragment was cut and gel isolated. A: 22 ng/µl, B: 25 ng/µl
Generation of DelH Plasmid 26-07
Analysis of G0, G1 & G2b on Gel
| Fragment | Size | Amount used [µl] | Amount used [ng] (by view) | Left for Gibson [µl] = [ng] | Concentration [ng/µl] |
|---|---|---|---|---|---|
| G0 | 18 Kb | 8 | 5 | 52 = 35.5 | 0.68 |
| G1 | 14 Kb | 8 | 10 | 12 = 15 | 1.25 |
| G2b | 5 Kb | 8 | 10 | 12 = 15 | 1.25 |
Overview on Fragments
| Fragment | Concentration [ng/µl] |
|---|---|
| G0 | 0.68 |
| G1 | 1.25 |
| G2b | 1.25 |
| BB A | 22 |
| BB B | 25 |
Gibson Assembly
| Assembly # | Fragments | Amount used [µl] |
|---|---|---|
| 1 |
|
|
| 2 |
|
|
| 3 |
|
|
- Incubated 1 hour at 50°C
- Stored ON at -20°C
Purification of Gibson Mix
- Purification performed with the long range gel extraction kit (because of 23 Kb size) of the entire sample (20 µl).
- Performed the purification by calculating with 100 mg DNA (gel- step1)
- Washing step with QX1 was not performed, because it is only to remove the residual agarose and we don't want to loose any DNA.
Electroporation
- Electroporation into freshly prepared electrocompetent cell following the protocol
- Streaked on LB Amp plates and incubated ON at 37°C
Result
There were no colonies on any of the plates. Ginson assembly and electroporation have to be repeated.
Generation of DelH Plasmid 28-07
NEB Protocol for Gibson Assembly
- Optimized cloning efficiency is 50–100 ng of vectors with 2–3 fold of excess inserts. Use 5 times more of inserts if size is less than 200 bps. Optimized cloning efficiency is 50-100 ng of vectors with 2-3 fold of excess inserts. Use 5 times more of inserts if size is less than 200 bps.
- Electrocompetent Cells Transformation Protocol:
- Thaw electrocompetent cells on ice.
- Transfer 50 μl of electrocompetent cells to a pre-chilled electroporation cuvette with 1 mM gap.
- Dilute assembled products 3-fold with H2O prior electroporation. This can be achieved by mixing 5 μl of assembled products with 10 μl of H2O. Add 1 μ l of the diluted assembly product to electrocompetent cells.Mix gently by pipetting up and down or flicking the tube 4–5 times. Do not vortex. Place the mixture on ice for 30 minutes. Do not mix.
- Mix gently by pipetting up.
- Once DNA is added to the cells, electroporation can be carried out immediately. It is not necessary to incubate DNA with cells.Add 950 μl of room temperature SOC media* to tubes.
- Add 950 μl of room temperature SOC media to the cuvette immediately after electroporation.
- Place the tube at 37°C for 60 minutes. Shake vigorously (250 rpm) or rotate.
- Warm selection plates to 37°C.
- Spread 100 μl of the cells onto the plates.
- Incubate overnight at 37°C.
Gibson Assembly
| Fragments | Concentraion [ng/µl] | Amount [µl] |
|---|---|---|
| G0 | 0.68 | 9 |
| BB (8.8) | 22 | 1 |
| Gibson Master Mix | 2x | 10 |
- Incubated 1 h at 37 °C in thermocycler
- 5 µl of mix added to 10 µl ddH2O and stored at -20°C
- 10 µl stored for isoprop purification
Result
Remaining 5 µl were checked on a gel:
Expected band: 23 Kb
No product visible of correct size. There is a light band at ~1.5 Kb.
- => Most probably too little DNA loaded.
Electroporation
- Electroporation into freshly prepared electrocompetent cell following the protocol
- Streaked on LB Amp plates and incubated ON at 37°C
Result
There were few colonies on the plates. Red colonies were picked and screenied by colony-PCR, but none of them was positive. The Gibson assembly has to be repeated.
Preparation of Electrocompetent E.coli DH10ß
According to the protocol
Amplification of Backbone pSB6A1-lacZ-mRFP
Test Restriction Digest
| Fragment | DNA [µl] | H2O [µl] | Enzymes [µl] | Buffer 3.1 [µl] |
|---|---|---|---|---|
| BB A (22 ng/µl) | 8 = 200 ng | 9 | NotI: 1 | 2 |
- Incubated 1.5 h at 37 °C
Result
Expected bands: 4 Kb & 1 Kb
Gel shows shows expeted bands at 4 Kb and 1 Kb.
- => Backbone is fine.
 "
"