Team:Heidelberg/Templates/DelH week14
From 2013.igem.org
m |
m (→Colony-PCR Conditions CP.W14.C) |
||
| (2 intermediate revisions not shown) | |||
| Line 109: | Line 109: | ||
{| class="wikitable" style="float:left; margin-right:1em" | {| class="wikitable" style="float:left; margin-right:1em" | ||
|- | |- | ||
| - | ! Reagent !! Electr. 1 !! Electr. 1 !! Electr. 1 !! Electr. 2 !! Electr. 2!! Electr. 2!! Electr. 2 | + | ! Reagent !! Electr. 1 !! Electr. 1 !! Electr. 1 !! Electr. 2 !! Electr. 2!! Electr. 2!! Electr. 2 |
|- | |- | ||
| - | | Expected length [bp] || 663 || 663 || 663 || 663 || 663 || 663 || 663 | + | | Expected length [bp] || 663 || 663 || 663 || 663 || 663 || 663 || 663 |
|- | |- | ||
| - | | Named || 1a || 1b || 1c || 2a || 2b || 2c || 2d | + | | Named || 1a || 1b || 1c || 2a || 2b || 2c || 2d |
|- | |- | ||
| - | | Template || 5 colonies of E1 || 5 colonies of E1 || 5 colonies of E1 || 5 colonies of E2 || 5 colonies of E2 || 5 colonies of E2 || 5 colonies of E2 | + | | Template || 5 colonies of E1 || 5 colonies of E1 || 5 colonies of E1 || 5 colonies of E2 || 5 colonies of E2 || 5 colonies of E2 || 5 colonies of E2 |
|- | |- | ||
| - | | Primer fw 10 µM || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 | + | | Primer fw 10 µM || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 |
|- | |- | ||
| - | | Primer rev 10 µM || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev||2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl | + | | Primer rev 10 µM || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev||2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev |
|- | |- | ||
| - | | Dream-Taq Polymerase (2x) || 10 µl || 10 µl || 10 µl || 10 µl || 10 µl || 10 µl | + | | Dream-Taq Polymerase (2x) || 10 µl || 10 µl || 10 µl || 10 µl || 10 µl || 10 µl || 10 µl |
|- | |- | ||
| - | | ddH<sub>2</sub>O || 6 µl || 6 µl || 6µl || 6 µl || 6 µl || 6 µl || 6 µl || 6 µl || 6 µl || 6 µl || 6 µl || 6 µl|| 6µl || 6 µl || 6 µl || 6 µl || 6 µl || 6 µl|| 6 µl || 6 µl || 6 µl || 6 µl || 6 µl | + | | ddH<sub>2</sub>O || 6 µl || 6 µl || 6µl || 6 µl || 6 µl || 6 µl || 6 µl |
| + | |} | ||
| + | {| class="wikitable" style="float:left; margin-right:1em" | ||
| + | |- | ||
| + | ! Reagent !! Electr. 3 !! Electr. 3 !! Electr. 3 !! Electr. 3 !! Electr. 4 !! Electr. 4 !! Electr. 4 !! Electr. 4 | ||
| + | |- | ||
| + | | Expected length [bp] || 663 || 663 || 663 || 663 || 663 || 663 || 663 || 663 | ||
| + | |- | ||
| + | | Named || 3a || 3b || 3c || 3d || 4a || 4b || 4c || 4d | ||
| + | |- | ||
| + | | Template || 5 colonies of E3 || 5 colonies of E3 || 5 colonies of E3 || 5 colonies of E3 || 5 colonies of E4 || 5 colonies of E4 || 5 colonies of E4 || 5 colonies of E4 | ||
| + | |- | ||
| + | | Primer fw 10 µM || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 | ||
| + | |- | ||
| + | | Primer rev 10 µM || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev ||2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev | ||
| + | |- | ||
| + | | Dream-Taq Polymerase (2x) || 10 µl || 10 µl || 10 µl || 10 µl || 10 µl || 10 µl || 10 µl || 10 µl | ||
| + | |- | ||
| + | | ddH<sub>2</sub>O || 6 µl || 6 µl || 6 µl || 6 µl || 6 µl|| 6µl || 6 µl || 6 µl | ||
| + | |} | ||
| + | {| class="wikitable" style="float:left; margin-right:1em" | ||
| + | |- | ||
| + | ! Reagent !! Electr. 5 !! Electr. 5 !! Electr. 5 !! Electr. 5 !! Electr. 6 !! Electr. 6 !! Electr. 6 !! Electr. 6 | ||
| + | |- | ||
| + | | Expected length [bp] || 663 || 663 || 663|| 663 || 663 || 663 || 663 || 663 | ||
| + | |- | ||
| + | | Named || 5a || 5b || 5c || 5d || 6a || 6b || 6c || 6d | ||
| + | |- | ||
| + | | Template || 5 colonies of E5 || 5 colonies of E5 || 5 colonies of E5 || 5 colonies of E5 || 5 colonies of E6 || 5 colonies of E6 || 5 colonies of E6 || 5 colonies of E6 | ||
| + | |- | ||
| + | | Primer fw 10 µM || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 | ||
| + | |- | ||
| + | | Primer rev 10 µM || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev | ||
| + | |- | ||
| + | | Dream-Taq Polymerase (2x) || 10 µl || 10 µl || 10 µl || 10 µl || 10 µl || 10 µl || 10 µl || 10 µl | ||
| + | |- | ||
| + | | ddH<sub>2</sub>O || 6 µl || 6 µl || 6 µl|| 6 µl || 6 µl || 6 µl || 6 µl || 6 µl | ||
|} | |} | ||
{| class="wikitable" style="float:left" | {| class="wikitable" style="float:left" | ||
| Line 145: | Line 181: | ||
| 1 || 12 || inf | | 1 || 12 || inf | ||
|} | |} | ||
| + | |||
| + | |||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
| + | |||
====Result==== | ====Result==== | ||
Expected band: 663 bp | Expected band: 663 bp | ||
| Line 160: | Line 199: | ||
{| class="wikitable" style="float:left; margin-right:1em" | {| class="wikitable" style="float:left; margin-right:1em" | ||
|- | |- | ||
| - | ! Reagent !! 3c !! 3c !! 3c !! 3c !! 3c !! 4a !! 4a !! 4a !! 4a !! 4a !! 6b !! 6b !! 6b !! 6b !! 6b | + | ! Reagent !! 3c !! 3c !! 3c !! 3c !! 3c |
| + | |- | ||
| + | | Expected length [bp] || 663 || 663 || 663 || 663 || 663 | ||
| + | |- | ||
| + | | Named || 3c 1 || 3c 2 || 3c 3 || 3c 4 || 3c 5 | ||
| + | |- | ||
| + | | Template (1 µl) || 1 colonies of E3c || 1 colonies of E3c|| 1 colonies of E3c|| 1 colonies of E3c|| 1 colonies of E3c | ||
| + | |- | ||
| + | | Primer fw 10 µM || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 | ||
| + | |- | ||
| + | | Primer rev 10 µM || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev||2 µl Screen_delH_rev || 2 µl Screen_delH_rev | ||
| + | |- | ||
| + | | Dream-Taq Polymerase (2x) || 10 µl || 10 µl || 10 µl || 10 µl || 10 µl | ||
| + | |- | ||
| + | | ddH<sub>2</sub>O || 5 µl || 5 µl || 5µl || 5 µl || 5 µl | ||
| + | |} | ||
| + | {| class="wikitable" style="float:left; margin-right:1em" | ||
| + | |- | ||
| + | ! Reagent !! 4a !! 4a !! 4a !! 4a !! 4a | ||
| + | |- | ||
| + | | Expected length [bp] || 663 || 663 || 663 || 663 || 663 | ||
| + | |- | ||
| + | | Named || 4a 1 || 4a 2 || 4a 3 || 4a 4 || 4a 5 | ||
| + | |- | ||
| + | | Template (1 µl) || 1 colonies of E4a ||1 colonies of E4a ||1 colonies of E4a ||1 colonies of E4a ||1 colonies of E4a | ||
| + | |- | ||
| + | | Primer fw 10 µM || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 | ||
| + | |- | ||
| + | | Primer rev 10 µM || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev | ||
| + | |- | ||
| + | | Dream-Taq Polymerase (2x) || 10 µl || 10 µl || 10 µl || 10 µl || 10 µl | ||
| + | |- | ||
| + | | ddH<sub>2</sub>O || 5 µl || 5 µl || 5 µl || 5 µl || 5 µl | ||
| + | |} | ||
| + | {| class="wikitable" style="float:left; margin-right:1em" | ||
| + | |- | ||
| + | ! Reagent !! 6b !! 6b !! 6b !! 6b !! 6b | ||
|- | |- | ||
| - | | Expected length [bp] | + | | Expected length [bp] || 663 || 663 || 663 || 663 || 663 |
|- | |- | ||
| - | | Named | + | | Named ||6b 1 || 6b 2 || 6b 3 || 6b 4 || 6b 5 |
|- | |- | ||
| - | | Template (1 µl) | + | | Template (1 µl) || 1 colonies of E6b || 1 colonies of E6b|| 1 colonies of E6b|| 1 colonies of E6b|| 1 colonies of E6b |
|- | |- | ||
| - | | Primer fw 10 µM | + | | Primer fw 10 µM || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 || 2 µl VF2 |
|- | |- | ||
| - | | Primer rev 10 µM || | + | | Primer rev 10 µM || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev || 2 µl Screen_delH_rev |
|- | |- | ||
| - | | Dream-Taq Polymerase (2x) | + | | Dream-Taq Polymerase (2x) || 10 µl || 10 µl || 10 µl || 10 µl || 10 µl |
|- | |- | ||
| - | | ddH<sub>2</sub>O | + | | ddH<sub>2</sub>O || 5 µl || 5 µl|| 5µl || 5 µl || 5 µl |
|} | |} | ||
{| class="wikitable" style="float:left" | {| class="wikitable" style="float:left" | ||
| Line 197: | Line 272: | ||
|} | |} | ||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
| + | |||
====Result==== | ====Result==== | ||
Expected band: 663 bp | Expected band: 663 bp | ||
Latest revision as of 11:30, 25 October 2013
Contents |
29-07 - 04-08-13
Generation of DelH Plasmid 28-07
Purification of Gibson Mix
10 µl of the Gibson mix were isopropanol purified as described in the protocol.
5 µl of the Gibson reaction were mixed with 10 µl ddH2O.
Electroporation
- Electroporation into freshly prepared electrocompetent cell following the protocol.
| E1 | E2 | E3 |
|---|---|---|
| 5 µl of Gibson + 10 µl H2O | 10 µl of isoprop purified Gibson assembly | no DNA |
- Grow 0.5 h with 400 µl SOC at 37°C
- Add 9 ml LB Amp and incubate 3 h at 37°C
- Incubate ON at 37°C on LB Amp plate as well as LB (no Amp) for control purposes
Result
There were few colonies on the plates. Red colonies were picked and screenied by colony-PCR, but none of them was positive. The Gibson assembly has to be repeated.
Generation of DelH Plasmid 01-08
Gibson Assembly
| Fragment | Concentraion | Amount used [µl] (2 vials) | Amount used [µl] |
|---|---|---|---|
| G0 | 0,68 ng/µl | 9 | 6 |
| BB (8.8) | 22 ng/µl | 1 | 4 |
| Gibson Master Mix | 2x | 10 | 10 |
- Incubated 1 h at 50 °C in thermocycler
Electroporation
| Electroporation # | Mix # | Amount of Mix [µl] | ddH2O [µl] | Isopropanol | Amount electroporated [µl] |
|---|---|---|---|---|---|
| 1 | 1 | 10 | 20 | - | 1 |
| 2 | 1 | 10 | 20 | - | 14 |
| 3 | 1 | 30 | - | yes | 20 |
| 4 | 2 | 5 | 10 | - | 1 |
| 5 | 2 | 5 | 10 | - | 29 |
| 6 | 2 | 15 | - | yes | 20 |
| 7 | - | - | 10 | - | 10 |
- Plated on one LB agar plate each
- Stored ON at 37°C
Colony-PCR Conditions CP.W14.A
| Reagent | Electroporation 1 | Electroporation 2 | Electroporation 3 | Electroporation 4 | Electroporation 5 | Electroporation 6 |
|---|---|---|---|---|---|---|
| Expected length [bp] | 663 | 663 | 663 | 663 | 663 | 663 |
| Named | 1 | 2 | 3 | 4 | 5 | 6 |
| Template | 5 colonies of E1 | 5 colonies of E2 | 5 colonies of E3 | 5 colonies of E4 | 5 colonies of E5 | 5 colonies of E6 |
| Primer fw 10 µM | 1 µl VF2 | 1 µl VF2 | 1 µl VF2 | 1 µl VF2 | 1 µl VF2 | 1 µl VF2 |
| Primer rev 10 µM | 1 µl Screen_delH_rev | 1 µl Screen_delH_rev | 1 µl Screen_delH_rev | 1 µl Screen_delH_rev | 1 µl Screen_delH_rev | 1 µl Screen_delH_rev |
| Dream-Taq Polymerase (2x) | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl |
| ddH2O | 8 µl | 8 µl | 8 µl | 8 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 95 | 120 |
| 12 | 95 | 60 |
| 68 (touchdown -0.5°C) | 30 | |
| 72 | 45 | |
| 18 | 95 | 60 |
| 65 (touchdown -0.5°C) | 30 | |
| 72 | 45 | |
| 1 | 12 | inf |
Result
Expected band: 663 bp
Gel does not show any band.
- => None of the analyzed colonies is positive.
Colony-PCR Conditions CP.W14.B
Picked 20 colonies per plate and 5 colonies per PCR tube.
| Reagent | Electr. 1 | Electr. 1 | Electr. 1 | Electr. 2 | Electr. 2 | Electr. 2 | Electr. 2 |
|---|---|---|---|---|---|---|---|
| Expected length [bp] | 663 | 663 | 663 | 663 | 663 | 663 | 663 |
| Named | 1a | 1b | 1c | 2a | 2b | 2c | 2d |
| Template | 5 colonies of E1 | 5 colonies of E1 | 5 colonies of E1 | 5 colonies of E2 | 5 colonies of E2 | 5 colonies of E2 | 5 colonies of E2 |
| Primer fw 10 µM | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 |
| Primer rev 10 µM | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev |
| Dream-Taq Polymerase (2x) | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl |
| ddH2O | 6 µl | 6 µl | 6µl | 6 µl | 6 µl | 6 µl | 6 µl |
| Reagent | Electr. 3 | Electr. 3 | Electr. 3 | Electr. 3 | Electr. 4 | Electr. 4 | Electr. 4 | Electr. 4 |
|---|---|---|---|---|---|---|---|---|
| Expected length [bp] | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 |
| Named | 3a | 3b | 3c | 3d | 4a | 4b | 4c | 4d |
| Template | 5 colonies of E3 | 5 colonies of E3 | 5 colonies of E3 | 5 colonies of E3 | 5 colonies of E4 | 5 colonies of E4 | 5 colonies of E4 | 5 colonies of E4 |
| Primer fw 10 µM | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 |
| Primer rev 10 µM | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev |
| Dream-Taq Polymerase (2x) | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl |
| ddH2O | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6µl | 6 µl | 6 µl |
| Reagent | Electr. 5 | Electr. 5 | Electr. 5 | Electr. 5 | Electr. 6 | Electr. 6 | Electr. 6 | Electr. 6 |
|---|---|---|---|---|---|---|---|---|
| Expected length [bp] | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 |
| Named | 5a | 5b | 5c | 5d | 6a | 6b | 6c | 6d |
| Template | 5 colonies of E5 | 5 colonies of E5 | 5 colonies of E5 | 5 colonies of E5 | 5 colonies of E6 | 5 colonies of E6 | 5 colonies of E6 | 5 colonies of E6 |
| Primer fw 10 µM | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 |
| Primer rev 10 µM | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev |
| Dream-Taq Polymerase (2x) | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl |
| ddH2O | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl |
| Cycles | Temperature DelH-G0 [°C] | Time [s] |
|---|---|---|
| 1 | 95 | 120 |
| 12 | 95 | 60 |
| 68 (touchdown -0.5°C) | 30 | |
| 72 | 45 | |
| 18 | 95 | 60 |
| 65 (touchdown -0.5°C) | 30 | |
| 72 | 45 | |
| 1 | 12 | inf |
Result
Expected band: 663 bp

l1: 2log, l2-5: 20 picked colonies from plate 4: every PCR tube has 5 colonies, l6-9:20 picked colonies from plate 5every PCR tube has 5 colonies,l10-14:20 picked colonies from plate 6 every PCR tube has 5 colonies
bands on lanes 4a, 4b, 6a, 6b, 6d at the expected 663 bp, so the next step is making PCRs of 1 colony per PCR of these probes

l1-3:3 picked colonies from plate 1, every PCR tube has 5 colonies, l4-7:4 picked colonies from plate 2, every PCR tube has 5 colonies,l8: 2log,l9-13:4 picked colonies from plate 3 every PCR tube has 5 colonies
bands on lanes 3b, 3c, 3d, 4a, 4b, 6a, 6b, 6d at the expected 663 bp, so the next step is making PCRs of 1 colony per PCR of these probes
Gel shows bands for b, 3c, 3d, 4a, 4b, 6a, 6b, 6d at the expected 663 bp.
- => Make single colony-PCRs.
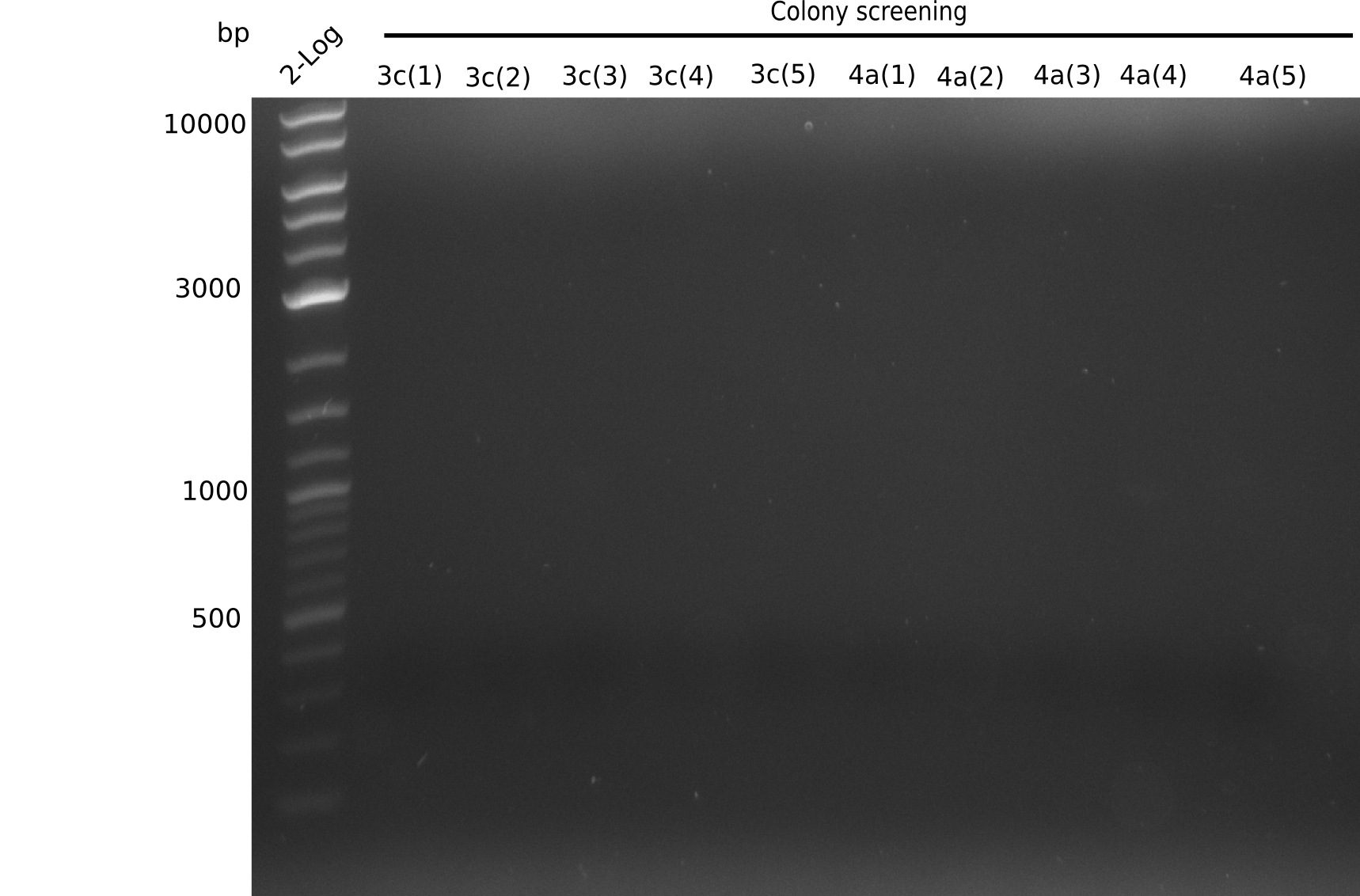
Colony-PCR Conditions CP.W14.C
1 µl of colony ON culture picked
| Reagent | 3c | 3c | 3c | 3c | 3c |
|---|---|---|---|---|---|
| Expected length [bp] | 663 | 663 | 663 | 663 | 663 |
| Named | 3c 1 | 3c 2 | 3c 3 | 3c 4 | 3c 5 |
| Template (1 µl) | 1 colonies of E3c | 1 colonies of E3c | 1 colonies of E3c | 1 colonies of E3c | 1 colonies of E3c |
| Primer fw 10 µM | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 |
| Primer rev 10 µM | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev |
| Dream-Taq Polymerase (2x) | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl |
| ddH2O | 5 µl | 5 µl | 5µl | 5 µl | 5 µl |
| Reagent | 4a | 4a | 4a | 4a | 4a |
|---|---|---|---|---|---|
| Expected length [bp] | 663 | 663 | 663 | 663 | 663 |
| Named | 4a 1 | 4a 2 | 4a 3 | 4a 4 | 4a 5 |
| Template (1 µl) | 1 colonies of E4a | 1 colonies of E4a | 1 colonies of E4a | 1 colonies of E4a | 1 colonies of E4a |
| Primer fw 10 µM | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 |
| Primer rev 10 µM | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev |
| Dream-Taq Polymerase (2x) | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl |
| ddH2O | 5 µl | 5 µl | 5 µl | 5 µl | 5 µl |
| Reagent | 6b | 6b | 6b | 6b | 6b |
|---|---|---|---|---|---|
| Expected length [bp] | 663 | 663 | 663 | 663 | 663 |
| Named | 6b 1 | 6b 2 | 6b 3 | 6b 4 | 6b 5 |
| Template (1 µl) | 1 colonies of E6b | 1 colonies of E6b | 1 colonies of E6b | 1 colonies of E6b | 1 colonies of E6b |
| Primer fw 10 µM | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 |
| Primer rev 10 µM | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev |
| Dream-Taq Polymerase (2x) | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl |
| ddH2O | 5 µl | 5 µl | 5µl | 5 µl | 5 µl |
| Cycles | Temperature DelH-G0 [°C] | Time [s] |
|---|---|---|
| 1 | 95 | 120 |
| 12 | 95 | 60 |
| 68 (touchdown -0.5°C) | 30 | |
| 72 | 45 | |
| 18 | 95 | 60 |
| 65 (touchdown -0.5°C) | 30 | |
| 72 | 45 | |
| 1 | 12 | inf |
Result
Expected band: 663 bp
- => No band is visible. There should be a band at at least one of each sample (for example 6B), because in the previous PCR we observed a band.
Because of the explained reason above we incubated 40 colonies seperately which might be positive ON at 37°C in 200 µl LB Amp. 200 µl of the ON cultire was mixed with 1.8 ml LB Amp for five colonies of each probe: 3b, 3c, 3d, 4a, 4b, 6a, 6b, 6d.
 "
"