Team:TU-Eindhoven/Results
From 2013.igem.org
(→Aerobic Expression) |
(→Anaerobic FNR Testing) |
||
| (7 intermediate revisions not shown) | |||
| Line 6: | Line 6: | ||
== CEST Agent Testing == | == CEST Agent Testing == | ||
| - | To evaluate all the potential CEST contrast agents, MRI measurements were carried out and analyzed as described in [[Team:TU-Eindhoven/MRIProcessing | MRI Data Processing]]. The most promising agents seemed to be the lysine based contrast agents. All compounds that have a high lysine percentage | + | To evaluate all the potential CEST contrast agents, MRI measurements were carried out and analyzed as described in [[Team:TU-Eindhoven/MRIProcessing | MRI Data Processing]]. The most promising agents seemed to be the lysine based contrast agents. All compounds that have a high lysine percentage expressed lysine based CEST contrast. The best distinguishable agent was ''1PJN'' ([http://parts.igem.org/Part:BBa_K1123015 BBa_K1123015]), based on the graph below ({{:Team:TU-Eindhoven/Template:Figure | id=1PJNMRIPlot }}). |
{{:Team:TU-Eindhoven/Template:Float | position=left | size=12 }} | {{:Team:TU-Eindhoven/Template:Float | position=left | size=12 }} | ||
| Line 13: | Line 13: | ||
== Anaerobic FNR Testing == | == Anaerobic FNR Testing == | ||
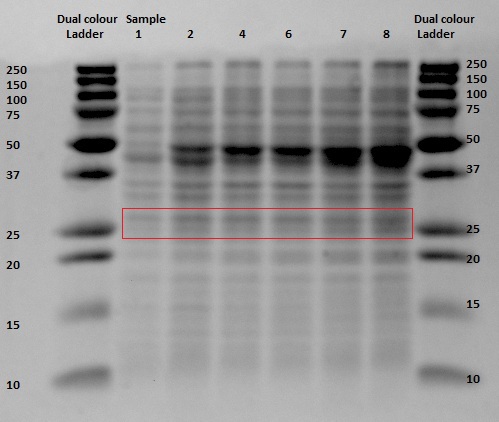
| - | After analyzing the samples taken during anaerobically induced expression of EGFP, we can conclude that the FNR promoter is a functional Biobrick. | + | After analyzing the samples taken during anaerobically induced expression of EGFP, we can conclude that the FNR promoter is a functional Biobrick. This conclusion was made after viewing the understanding gels and noticing the increased intensity of the bands related to the EGFP protein. |
{{:Team:TU-Eindhoven/Template:ImageList}} | {{:Team:TU-Eindhoven/Template:ImageList}} | ||
{{:Team:TU-Eindhoven/Template:Float | position=left | size=6 }} | {{:Team:TU-Eindhoven/Template:Float | position=left | size=6 }} | ||
| Line 24: | Line 24: | ||
{{:Team:TU-Eindhoven/Template:ImageListEnd}} | {{:Team:TU-Eindhoven/Template:ImageListEnd}} | ||
| - | + | To strengthen this argument a Western Blot was performed allowing us to bind antibodies directly to EGFP. By performing the necessary washing steps and staining the following blot membrane was found. | |
| + | |||
| + | {{:Team:TU-Eindhoven/Template:ImageList}} | ||
| + | {{:Team:TU-Eindhoven/Template:Float | position=left | size=9 }} | ||
| + | {{:Team:TU-Eindhoven/Template:Image | filename=WesternBlot.png}} | ||
| + | {{:Team:TU-Eindhoven/Template:FloatEnd | caption=Western Blot performed on purified samples of EGFP expressed anaerobically. C- is a negative control, a purified sample of culture medium allowed to house EGFP expressing bacteria in an aerobic environment. C+ is a positive control of EGFP bound to a CNA35 protein. The first 6 samples t1 to t6 were expressed anaerobically at 0% Oxygen whereas the second set of 6 samples t1 to t6 were expressed at 5% Oxygen | id=WesternBlot }} | ||
| + | {{:Team:TU-Eindhoven/Template:ImageListEnd}} | ||
| + | |||
| + | In {{:Team:TU-Eindhoven/Template:Figure | id=WesternBlot }} it becomes clear that EGFP is being produced as only EGFP proteins will bind to the EGFP specific antibodies. In {{:Team:TU-Eindhoven/Template:Figure | id=WesternBlot }} we also note the disappearance of all other bands on the Western Blot. | ||
| + | |||
| + | Furthermore we also performed fluorescence measurements of the samples taken at different time points during the anaerobically induced expression of EGFP. These can be viewed below. | ||
{{:Team:TU-Eindhoven/Template:ImageList}} | {{:Team:TU-Eindhoven/Template:ImageList}} | ||
| Line 34: | Line 44: | ||
{{:Team:TU-Eindhoven/Template:FloatEnd | caption=Values of the peaks of the emission spectra of EGFP per sample | id=Emissionpeaks }} | {{:Team:TU-Eindhoven/Template:FloatEnd | caption=Values of the peaks of the emission spectra of EGFP per sample | id=Emissionpeaks }} | ||
{{:Team:TU-Eindhoven/Template:ImageListEnd}} | {{:Team:TU-Eindhoven/Template:ImageListEnd}} | ||
| - | These experiments resulted in the | + | What becomes clear from the fluorescence measurements is that the amount of EGFP is indeed increasing in accordance with increases in the time. (Which also confirms that the low intensity bands seen in the gels above were in fact EGFP). These experiments resulted in the conclusion that we have two more functional Biobricks: [http://parts.igem.org/wiki/index.php?title=Part:BBa_K1123000 BBa_K1123001] and [http://parts.igem.org/wiki/index.php?title=Part:BBa_K1123005 BBa_K1123005]. |
To see detailed information and results, see [https://2013.igem.org/Team:TU-Eindhoven/AnearobicTesting Anaerobic FNR Testing]. | To see detailed information and results, see [https://2013.igem.org/Team:TU-Eindhoven/AnearobicTesting Anaerobic FNR Testing]. | ||
== General Result == | == General Result == | ||
| - | As a general result of all the experiments we have a promising CEST contrast | + | As a general result of all the experiments, we have obtained a promising CEST contrast agent alongside a functional FNR promoter, which can be induced anaerobically at 37°C. When exposed to hypoxic conditions for longer periods of time we also notice an increase in the amount of protein being produced, as we would expect. |
| - | Overall, we showed that all the | + | Overall, we showed that all the separate mechanisms are working correctly and that a combined construct is still functional. This proof of concept is promising, but further experiments, such as ''in vivo'' experiments and experiments to ensure the killing mechanism works, need to be performed to say whether this could really be used for tumor targeting within the human body. |
{{:Team:TU-Eindhoven/Template:BaseFooter}} | {{:Team:TU-Eindhoven/Template:BaseFooter}} | ||
Latest revision as of 15:01, 26 October 2013



Contents |
Main Results
Aerobic Expression
In the experiments for the aerobic expression of the proteins, we saw that we were able to produce a number of proteins correctly. The proteins 1ETF, 1PJN, 1G70 and EGFP were visibly produced and purified by means of their His-Tag and/or exclusion body extraction. The other proteins we had designed were not seen as clearly, many not all, after expression, however, for most of these proteins, we did end up seeing CEST contrast. We can conclude that the following Biobricks were expressed most succesfully: [http://parts.igem.org/Part:BBa_K1123014 BBa_K11230014], [http://parts.igem.org/Part:BBa_K1123015 BBa_K1123015], [http://parts.igem.org/Part:BBa_K1123016 BBa_K1123016] and [http://parts.igem.org/Part:BBa_K1123017 BBa_K1123017]. For more detailed information and further results obtained during these experiments, see CEST Agent Testing.
CEST Agent Testing
To evaluate all the potential CEST contrast agents, MRI measurements were carried out and analyzed as described in MRI Data Processing. The most promising agents seemed to be the lysine based contrast agents. All compounds that have a high lysine percentage expressed lysine based CEST contrast. The best distinguishable agent was 1PJN ([http://parts.igem.org/Part:BBa_K1123015 BBa_K1123015]), based on the graph below ().

Anaerobic FNR Testing
After analyzing the samples taken during anaerobically induced expression of EGFP, we can conclude that the FNR promoter is a functional Biobrick. This conclusion was made after viewing the understanding gels and noticing the increased intensity of the bands related to the EGFP protein.

To strengthen this argument a Western Blot was performed allowing us to bind antibodies directly to EGFP. By performing the necessary washing steps and staining the following blot membrane was found.
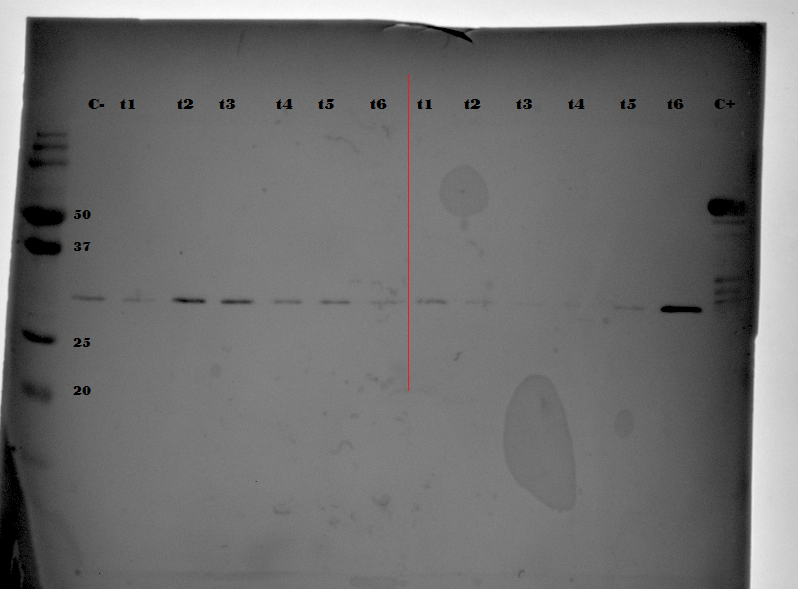
In it becomes clear that EGFP is being produced as only EGFP proteins will bind to the EGFP specific antibodies. In we also note the disappearance of all other bands on the Western Blot.
Furthermore we also performed fluorescence measurements of the samples taken at different time points during the anaerobically induced expression of EGFP. These can be viewed below.
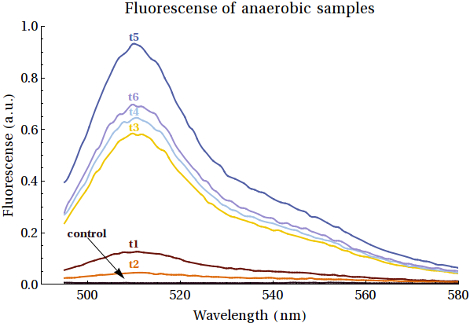
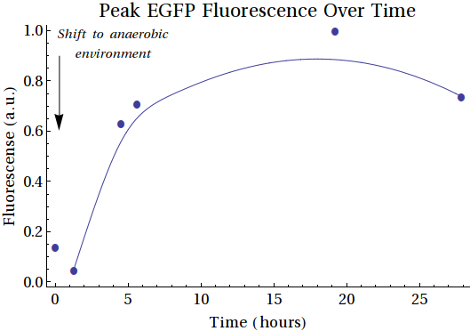
General Result
As a general result of all the experiments, we have obtained a promising CEST contrast agent alongside a functional FNR promoter, which can be induced anaerobically at 37°C. When exposed to hypoxic conditions for longer periods of time we also notice an increase in the amount of protein being produced, as we would expect. Overall, we showed that all the separate mechanisms are working correctly and that a combined construct is still functional. This proof of concept is promising, but further experiments, such as in vivo experiments and experiments to ensure the killing mechanism works, need to be performed to say whether this could really be used for tumor targeting within the human body.
 "
"



