Team:Braunschweig/Results
From 2013.igem.org
(Created page with "{{Template:Team:Braunschweig/HeadBraunschweig taskbar}} <html> <style> h1 {font-size:16px; font-weight:bold; text-decoration:none; border:none; display:inline} #contents { c...") |
|||
| (15 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Template:Team:Braunschweig/HeadBraunschweig taskbar}} | {{Template:Team:Braunschweig/HeadBraunschweig taskbar}} | ||
| - | |||
<html> | <html> | ||
| Line 26: | Line 25: | ||
dl.imgRight dd {margin:0; font-size:90%;} | dl.imgRight dd {margin:0; font-size:90%;} | ||
| + | dl.imgCenter { text-align: center;} | ||
| + | dl.imgCenter dd {margin:0 auto; font-size:90%;} | ||
</style> | </style> | ||
| + | |||
<div id="contents"> | <div id="contents"> | ||
| - | <h1> | + | <h1>Results</h1> |
| - | <img alt="linie rot 8pix hoch" src="https://static.igem.org/mediawiki/2013/0/07/Team_Braunschweig_Red_line.jpg" width="850" height="1" /></p> | + | <img alt="linie rot 8pix hoch" src="https://static.igem.org/mediawiki/2013/0/07/Team_Braunschweig_Red_line.jpg" width="850" height="1" /> |
| + | In our project we were able to assemble two functional devices (BBa_K1073034 and BBa_K1073035) using standard Biobricks from the registry.<br> | ||
| + | To establish a stable microbial consortium our engineered constructs consist of different operation units. We demonstrated the functionality of each unit by individual experiments:<br> | ||
| + | |||
| + | <img alt="grey line" src="https://static.igem.org/mediawiki/2013/4/4c/Braunschweig_grey_line.png" width="850" height="1" vspace="20"/> | ||
| + | |||
| + | <p style="font-size:14px; font-weight:bold; text-decoration:none; border:none; color:#be1e3c">1. Reporter-chromoproteins were successfully integrated into the final constructs</p> | ||
| + | <p>The included reporter-chromoproteins were visible to the naked eye in less than 24 h during incubation on agar plates or in liquid culture (fig. 1). The use of chromoproteins as reporter facilitated the distinction of the different bacterial strains we intended to cultivate in co-culture. Featured chromoproteins were amilGFP, eforRed and aeBlue which developed a yellowish, bright pink and dark blue color.<br><br> | ||
| + | <dl class="imgCenter"> | ||
| + | <dt><img alt="Chromoproteins" src="https://static.igem.org/mediawiki/parts/thumb/c/c1/Expressed_chromoproteins.png/450px-Expressed_chromoproteins.png" width="300" /> | ||
| + | </dt> | ||
| + | <dd>Fig. 1: Successful integration of reporter-chromoproteins into our constructs.<br> Liquid overnight cultures expressing the chromoproteins amilGFP (left), eforRed (middle), aeBlue (right).</dd></dl> <br> | ||
| + | However, eforRed and amilGFP can also be detected by fluorescence (fig. 2-4). | ||
| + | |||
| + | <dl class="imgCenter"> | ||
| + | <dt><a href="https://static.igem.org/mediawiki/2013/d/df/Braunschweig_eforRed_spektren_results.png"><img alt="eforRed spectra" src="https://static.igem.org/mediawiki/2013/d/df/Braunschweig_eforRed_spektren_results.png" width="600" /></a> | ||
| + | </dt> | ||
| + | <dd>Fig. 2: Absorption and emission spectra of eforRed.</dd></dl> <br> | ||
| + | |||
| + | <dl class="imgCenter"> | ||
| + | <dt><a href="https://static.igem.org/mediawiki/2013/1/11/Braunschweig_amilGFP_spektren_results.png"><img alt="amilGFP spectra" src="https://static.igem.org/mediawiki/2013/1/11/Braunschweig_amilGFP_spektren_results.png" width="600" /></a> | ||
| + | </dt> | ||
| + | <dd>Fig. 3: Absorption and emission spectra of amilGFP.</dd></dl> <br> | ||
| + | |||
| + | <dl class="imgCenter"> | ||
| + | <dt><img alt="amilGFP eforRed Fluorescence" src="https://static.igem.org/mediawiki/2013/9/90/Braunschweig_Fluoreszenzaufnahme_amilGFP_eforRed.png" width="300" /> | ||
| + | </dt> | ||
| + | <dd>Fig. 4: Bacteria expressing eforRed and amilGFP can clearly be distinguished in fluorescence microscopy.</dd></dl> <br></p> | ||
| + | |||
| + | <img alt="grey line" src="https://static.igem.org/mediawiki/2013/4/4c/Braunschweig_grey_line.png" width="850" height="1" vspace="20"/> | ||
| + | |||
| + | <p style="font-size:14px; font-weight:bold; text-decoration:none; border:none; color:#be1e3c">2. The inducibility of an ampicillin resistance was shown</p> | ||
| + | |||
| + | <p>We were able to induce cell growth on ampicillin supplemented medium by addition of synthetic inducers. The expression of beta-lactamase in BBa_K1073034 and BBa_K1073035 is dependent on the induction of the promoters Plas and Prhl. The corresponding transcription activator (LasR and RhlR) is coded in each construct and expressed constitutively. Upon addition of the inducers (N-3-oxododecanoyl homoserine lactone for LasR and N-butyryl homoserine lactone for RhlR) cell growth in ampicillin containing medium was enabled whereas no significant growth was observed in control cultures (fig. 5). The bump at hour 4 was highly reproducible although we are not sure what it is caused by. | ||
| + | <dl class="imgCenter"> | ||
| + | <dt><a href="https://static.igem.org/mediawiki/parts/thumb/9/98/Braunschweig_2013_-_JM109_pSB1C3_K1073034.jpg/480px-Braunschweig_2013_-_JM109_pSB1C3_K1073034.jpg"><img alt="Induction of Growth" src="https://static.igem.org/mediawiki/parts/thumb/9/98/Braunschweig_2013_-_JM109_pSB1C3_K1073034.jpg/480px-Braunschweig_2013_-_JM109_pSB1C3_K1073034.jpg" width="400" /></a><a href="https://static.igem.org/mediawiki/parts/thumb/e/e4/Braunschweig2013_Top10_pSB1C3_K1073035.jpg/500px-Braunschweig2013_Top10_pSB1C3_K1073035.jpg"><img alt="Induction of Growth" src="https://static.igem.org/mediawiki/parts/thumb/e/e4/Braunschweig2013_Top10_pSB1C3_K1073035.jpg/500px-Braunschweig2013_Top10_pSB1C3_K1073035.jpg" width="400" /></a> | ||
| + | </dt> | ||
| + | <dd>Fig. 5: Successful induction of cell growth on medium that was supplemented with Ampicillin. Cell growth of <i>E. coli</i> strains bearing BBa_K1073034 and BBa_K1073035<br> | ||
| + | in Ampicillin medium was induced by addition of the corresponding inducers. | ||
| + | Due to the promoter leakiness of P<sub>las</sub> 5 µM Clavulanic acid, a ß-lactamase inhibitor, | ||
| + | was added to the medium to overcome background expression of ß-lactamase by BBa_K1073034. </dd></dl> <br></p> | ||
| + | |||
| + | <img alt="grey line" src="https://static.igem.org/mediawiki/2013/4/4c/Braunschweig_grey_line.png" width="850" height="1" vspace="20"/> | ||
| + | |||
| + | |||
| + | <p style="font-size:14px; font-weight:bold; text-decoration:none; border:none; color:#be1e3c">3. Successful production of inducers with BBa_K1073034 and BBa_K1073035</p> | ||
| + | |||
| + | <p>Using specific homoserine lactone (HSL) reporter strains, we were able to confirm presence of inducers in culture-supernatant of <i>E. coli</i> strains bearing the devices BBa_K1073034 and BBa_K1073035. The HSL reporter strains make use of plasmids that have a luciferase cloned behind inducible promoters. When the corresponding inducers are present in the medium, the reporter strains develop a bioluminescence that can easily be detected (fig. 6). | ||
| + | |||
| + | <dl class="imgCenter"> | ||
| + | <dt><a href="https://static.igem.org/mediawiki/2013/7/7d/Braunschweig_Biolumineszenz.png"><img alt="Bioluminescence" src="https://static.igem.org/mediawiki/2013/7/7d/Braunschweig_Biolumineszenz.png" width="700" /></a> | ||
| + | </dt> | ||
| + | <dd>Fig. 6: HSL were detected in culture supernatants using specific repoter strains.<br> | ||
| + | The reporter strains were cultivated in sterile filtered supernatant of<br> <i>E. coli</i> JM109::pSB1C3-BBa_K1073034 and <i>E. coli</i> Top10F’::pSB1C3-BBa_K1073035.<br> After 3 h cultivation bioluminescence was measured.</dd></dl> <br></p> | ||
| + | |||
| + | <img alt="grey line" src="https://static.igem.org/mediawiki/2013/4/4c/Braunschweig_grey_line.png" width="850" height="1" vspace="20"/> | ||
| + | |||
| + | <p style="font-size:14px; font-weight:bold; text-decoration:none; border:none; color:#be1e3c">4. Crossinduction of the constructs BBa_K1073034 and BBa_K1073035 was proven.</p> | ||
| + | <p> The crossinduction of beta-lactamase in <i>E. coli</i> strains bearing the constructs BBa_K1073034 and BBa_K1073035 was proven (fig.7).<br><br> | ||
| + | <dl class="imgCenter"> | ||
| + | <dt><img alt="Agar diffusion test" src="https://static.igem.org/mediawiki/2013/7/72/Braunschweig_Agardiffusion.png" width="600" /> | ||
| + | </dt> | ||
| + | <dd>Fig. 7: Successful induction of cell growth on plates that were supplemented with ampicillin. Cell growth of <i>E. coli</i> strains bearing<br> BBa_K1073034 and BBa_K1073035 on ampicillin supplemented medium was induced in agar diffusion tests. <i>E. coli</i> strains bearing the constructs<br> BBa_K107303 and BBa_K1073035 were plated on agar plates supplemented with ampicillin. Filter platelets soaked with<br> sterile filtered supernatant of the complementing strain were applied to the agar plates. </dd></dl> <br></p> | ||
| + | |||
| + | <img alt="grey line" src="https://static.igem.org/mediawiki/2013/4/4c/Braunschweig_grey_line.png" width="850" height="1" vspace="20"/> | ||
| + | |||
| + | <p style="font-size:14px; font-weight:bold; text-decoration:none; border:none; color:#be1e3c">5. Regulating influence of BBa_K1073034 and BBa_K1073035 in mixed cultures was shown.</p> | ||
| + | <p>After 24h batch cultivation of cultures inoculated with different ratios of strains bearing BBa_K1073034 and BBa_K1073035 the composition was shown to be similar for all inoculation ratios on Ampicillin supplemented medium whereas culture compositions on Chloramphenicol supplemented medium remained different to a great extent (fig. 8). The chloramphenicol resistance is constitutively expressed in the vector backbone of the two constructs, whereas the Ampicillin resistance is under the control of the inducible promoter. Thus, growth regulating effects of BBa_K1073034 and BBa_K1073035 have to be only expected on ampicillin but not on chloramphenicol medium.<br><br> | ||
| + | |||
| + | <dl class="imgCenter"> | ||
| + | <dt><img alt="Mixed cultures" src="https://static.igem.org/mediawiki/2013/5/5a/Braunschweig_Mischkultur.png" width="500" /> | ||
| + | </dt> | ||
| + | <dd>Fig. 8: Regulatory effects of BBa_K1073034 and BBa_K1073035 in mixed cultures. Culture compositions remained different in unregulated cultures,<br> whereas all regulated cultures exhibited comparable culture compositions after 24 hours. Mixed cultures were inoculated in different ratios of<br> <i>E. coli</i> JM109:: pSB1C3-BBa_K1073034 and <i>E. coli</i> Top10F’::pSB1C3-BBa_K1073035. Unregulated growth was obtained in medium supplemented<br> with Chloramphenicol whereas regulated growth was obtained in medium supplemented with Ampicillin. To visualize the culture composition the mixed<br> cultures were pelleted in microtiter plates. </dd></dl></p> | ||
| + | |||
| + | </div> | ||
| + | |||
| + | |||
| + | |||
<div id="sponsors"> | <div id="sponsors"> | ||
<h1>Our sponsors</h1></p> | <h1>Our sponsors</h1></p> | ||
| - | <img alt="linie rot 8pix hoch" src="https://static.igem.org/mediawiki/2013/0/07/Team_Braunschweig_Red_line.jpg" width=" | + | <img alt="linie rot 8pix hoch" src="https://static.igem.org/mediawiki/2013/0/07/Team_Braunschweig_Red_line.jpg" width="850" height="1" /></p> |
| - | <img src="https://static.igem.org/mediawiki/2013/ | + | <img src="https://static.igem.org/mediawiki/2013/9/9e/SponsorenBS.png" width="875px" /></p> |
</div> | </div> | ||
</html> | </html> | ||
Latest revision as of 15:12, 27 October 2013

Results
To establish a stable microbial consortium our engineered constructs consist of different operation units. We demonstrated the functionality of each unit by individual experiments:
1. Reporter-chromoproteins were successfully integrated into the final constructs
The included reporter-chromoproteins were visible to the naked eye in less than 24 h during incubation on agar plates or in liquid culture (fig. 1). The use of chromoproteins as reporter facilitated the distinction of the different bacterial strains we intended to cultivate in co-culture. Featured chromoproteins were amilGFP, eforRed and aeBlue which developed a yellowish, bright pink and dark blue color.

- Fig. 1: Successful integration of reporter-chromoproteins into our constructs.
Liquid overnight cultures expressing the chromoproteins amilGFP (left), eforRed (middle), aeBlue (right).
However, eforRed and amilGFP can also be detected by fluorescence (fig. 2-4).

- Fig. 4: Bacteria expressing eforRed and amilGFP can clearly be distinguished in fluorescence microscopy.
2. The inducibility of an ampicillin resistance was shown
We were able to induce cell growth on ampicillin supplemented medium by addition of synthetic inducers. The expression of beta-lactamase in BBa_K1073034 and BBa_K1073035 is dependent on the induction of the promoters Plas and Prhl. The corresponding transcription activator (LasR and RhlR) is coded in each construct and expressed constitutively. Upon addition of the inducers (N-3-oxododecanoyl homoserine lactone for LasR and N-butyryl homoserine lactone for RhlR) cell growth in ampicillin containing medium was enabled whereas no significant growth was observed in control cultures (fig. 5). The bump at hour 4 was highly reproducible although we are not sure what it is caused by.


- Fig. 5: Successful induction of cell growth on medium that was supplemented with Ampicillin. Cell growth of E. coli strains bearing BBa_K1073034 and BBa_K1073035
in Ampicillin medium was induced by addition of the corresponding inducers. Due to the promoter leakiness of Plas 5 µM Clavulanic acid, a ß-lactamase inhibitor, was added to the medium to overcome background expression of ß-lactamase by BBa_K1073034.
3. Successful production of inducers with BBa_K1073034 and BBa_K1073035
Using specific homoserine lactone (HSL) reporter strains, we were able to confirm presence of inducers in culture-supernatant of E. coli strains bearing the devices BBa_K1073034 and BBa_K1073035. The HSL reporter strains make use of plasmids that have a luciferase cloned behind inducible promoters. When the corresponding inducers are present in the medium, the reporter strains develop a bioluminescence that can easily be detected (fig. 6).
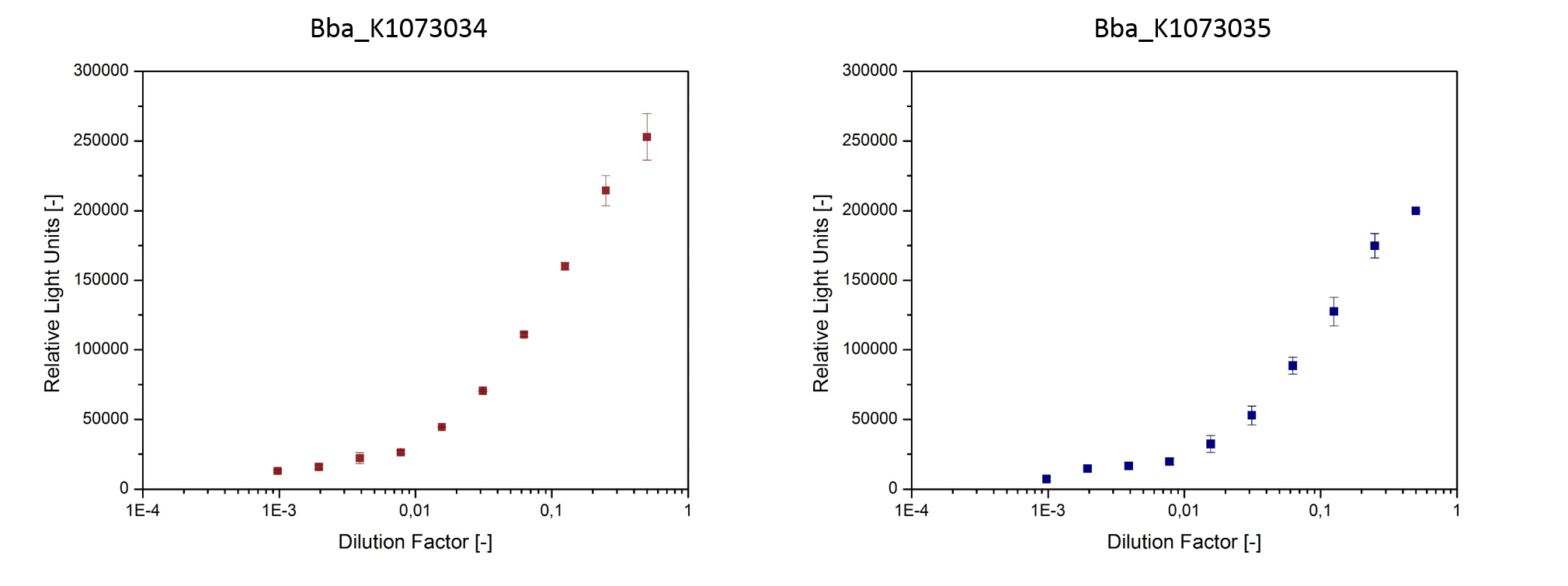
- Fig. 6: HSL were detected in culture supernatants using specific repoter strains.
The reporter strains were cultivated in sterile filtered supernatant of
E. coli JM109::pSB1C3-BBa_K1073034 and E. coli Top10F’::pSB1C3-BBa_K1073035.
After 3 h cultivation bioluminescence was measured.
4. Crossinduction of the constructs BBa_K1073034 and BBa_K1073035 was proven.
The crossinduction of beta-lactamase in E. coli strains bearing the constructs BBa_K1073034 and BBa_K1073035 was proven (fig.7).

- Fig. 7: Successful induction of cell growth on plates that were supplemented with ampicillin. Cell growth of E. coli strains bearing
BBa_K1073034 and BBa_K1073035 on ampicillin supplemented medium was induced in agar diffusion tests. E. coli strains bearing the constructs
BBa_K107303 and BBa_K1073035 were plated on agar plates supplemented with ampicillin. Filter platelets soaked with
sterile filtered supernatant of the complementing strain were applied to the agar plates.
5. Regulating influence of BBa_K1073034 and BBa_K1073035 in mixed cultures was shown.
After 24h batch cultivation of cultures inoculated with different ratios of strains bearing BBa_K1073034 and BBa_K1073035 the composition was shown to be similar for all inoculation ratios on Ampicillin supplemented medium whereas culture compositions on Chloramphenicol supplemented medium remained different to a great extent (fig. 8). The chloramphenicol resistance is constitutively expressed in the vector backbone of the two constructs, whereas the Ampicillin resistance is under the control of the inducible promoter. Thus, growth regulating effects of BBa_K1073034 and BBa_K1073035 have to be only expected on ampicillin but not on chloramphenicol medium.

- Fig. 8: Regulatory effects of BBa_K1073034 and BBa_K1073035 in mixed cultures. Culture compositions remained different in unregulated cultures,
whereas all regulated cultures exhibited comparable culture compositions after 24 hours. Mixed cultures were inoculated in different ratios of
E. coli JM109:: pSB1C3-BBa_K1073034 and E. coli Top10F’::pSB1C3-BBa_K1073035. Unregulated growth was obtained in medium supplemented
with Chloramphenicol whereas regulated growth was obtained in medium supplemented with Ampicillin. To visualize the culture composition the mixed
cultures were pelleted in microtiter plates.
Our sponsors

 "
"


