Team:HUST-China/Project
From 2013.igem.org
HUST Oshyn (Talk | contribs) |
HUST Oshyn (Talk | contribs) |
||
| Line 114: | Line 114: | ||
<h4>Propionate generation:</h4> | <h4>Propionate generation:</h4> | ||
<p>1.We firstly successfully copied the four genes ygfD/ygfH/ygfG/Sbm from E.coli strain K12, and then recombined them independently to expression vector. Purified protein from cell disruptions by Ni-chelating and did gel analysis. From figure-1 , we confirmed that the enzymes were successfully expressed.</p> | <p>1.We firstly successfully copied the four genes ygfD/ygfH/ygfG/Sbm from E.coli strain K12, and then recombined them independently to expression vector. Purified protein from cell disruptions by Ni-chelating and did gel analysis. From figure-1 , we confirmed that the enzymes were successfully expressed.</p> | ||
| - | <div><p><img src="https://static.igem.org/mediawiki/2013/6/6e/HUST-proj-res1.png" /></p> | + | <div style="text-align:center;"><p><img src="https://static.igem.org/mediawiki/2013/6/6e/HUST-proj-res1.png" /></p> |
<p>Figure1. SDS-PAGE analysis of Sbm, ygfD, ygfG, ygfH over-expression</p> | <p>Figure1. SDS-PAGE analysis of Sbm, ygfD, ygfG, ygfH over-expression</p> | ||
</div> | </div> | ||
<p>2.As we thought the overexpression of enzymes didn’t directly prove the increase of propionate, and we wanted to find the key gene in the reaction. So we used the positive clones to do fermentation. We firstly optimized the condition and found the best sampling time points. Then we used standard propionate of seven gradients to draw a linear graph between propionate concentration and HPLC peak area. Figure-2 shows the relationship.</p> | <p>2.As we thought the overexpression of enzymes didn’t directly prove the increase of propionate, and we wanted to find the key gene in the reaction. So we used the positive clones to do fermentation. We firstly optimized the condition and found the best sampling time points. Then we used standard propionate of seven gradients to draw a linear graph between propionate concentration and HPLC peak area. Figure-2 shows the relationship.</p> | ||
| - | <div><p><img src="https://static.igem.org/mediawiki/2013/7/76/HUST-proj-res2.png" /></p> | + | <div style="text-align:center;"><p><img src="https://static.igem.org/mediawiki/2013/7/76/HUST-proj-res2.png" /></p> |
<p>Figure-2. Standard curve of propionate and HPLC peak area</p> | <p>Figure-2. Standard curve of propionate and HPLC peak area</p> | ||
</div> | </div> | ||
<p>Later, we used the recombinant strain to do fermentation, and measured propionate concentration in the samples. Figure-3 shows the propionate increase percent of each gene recombinant strain. From the data, we found propionate production had a significant increase when ygfD transformed. In other words, we found the key gene- our oscillator’s output.</p> | <p>Later, we used the recombinant strain to do fermentation, and measured propionate concentration in the samples. Figure-3 shows the propionate increase percent of each gene recombinant strain. From the data, we found propionate production had a significant increase when ygfD transformed. In other words, we found the key gene- our oscillator’s output.</p> | ||
| - | <div><p><img src="https://static.igem.org/mediawiki/2013/0/05/HUST-proj-res3.png" /></p> | + | <div style="text-align:center;"><p><img src="https://static.igem.org/mediawiki/2013/0/05/HUST-proj-res3.png" /></p> |
<p>Figure-3. HPLC analysis wild-type BL21 and recombination BL21 with four genes</p> | <p>Figure-3. HPLC analysis wild-type BL21 and recombination BL21 with four genes</p> | ||
</div> | </div> | ||
| Line 128: | Line 128: | ||
<p>1.As for the oscillator, we first successfully constructed the two plasmids formed the dual-feedback circuit. (Circuit and plasmid were show in figure-4) LAA tag was added to the protein for rapid degradation. Gene sequencing confirmed the plasmids to be correct without any lethal mutation.</p> | <p>1.As for the oscillator, we first successfully constructed the two plasmids formed the dual-feedback circuit. (Circuit and plasmid were show in figure-4) LAA tag was added to the protein for rapid degradation. Gene sequencing confirmed the plasmids to be correct without any lethal mutation.</p> | ||
<div><p><img src="https://static.igem.org/mediawiki/2013/0/08/HUST-proj-res4.png" /></p> | <div><p><img src="https://static.igem.org/mediawiki/2013/0/08/HUST-proj-res4.png" /></p> | ||
| - | <p>Figure-4.The dual-feedback circuit and two plasmids we constructed</p> | + | <p style="text-align:center;">Figure-4.The dual-feedback circuit and two plasmids we constructed</p> |
</div> | </div> | ||
<p>2.After that, we transformed recombinant plasmid pET-28a (+) which had an mRFP reporter to check if it could function well. We induced the positive clone with 2mM IPTG and observed by fluorescence microscope. Figure-5 shows that it function well.</p> | <p>2.After that, we transformed recombinant plasmid pET-28a (+) which had an mRFP reporter to check if it could function well. We induced the positive clone with 2mM IPTG and observed by fluorescence microscope. Figure-5 shows that it function well.</p> | ||
<div><p><img src="https://static.igem.org/mediawiki/2013/8/83/HUST-proj-res5.png" /></p> | <div><p><img src="https://static.igem.org/mediawiki/2013/8/83/HUST-proj-res5.png" /></p> | ||
| - | <p>Figure-5. Fluorescence microscope photo of recombinant plasmid pET-28a (+) transformed cell induced by 2mM IPTG</p> | + | <p style="text-align:center;">Figure-5. Fluorescence microscope photo of recombinant plasmid pET-28a (+) transformed cell induced by 2mM IPTG</p> |
</div> | </div> | ||
<p>3.Later, we co-transformed two recombinant plasmids pET-28a (+) and pACYCDuet-1. After inducing positive clone by 0.7% Arabinose and 2 mM IPTG, we used fluorescence microscope to see RFP change of single cell and used fluorospectro photometer to see RFP change of multicells.Figure-6 & Figure-7 shows the result.</p> | <p>3.Later, we co-transformed two recombinant plasmids pET-28a (+) and pACYCDuet-1. After inducing positive clone by 0.7% Arabinose and 2 mM IPTG, we used fluorescence microscope to see RFP change of single cell and used fluorospectro photometer to see RFP change of multicells.Figure-6 & Figure-7 shows the result.</p> | ||
<div><p><img src="https://static.igem.org/mediawiki/2013/e/ec/HUST-proj-res6.png" /></p> | <div><p><img src="https://static.igem.org/mediawiki/2013/e/ec/HUST-proj-res6.png" /></p> | ||
| - | <p>Figure-6. Fluorescence change of single cell</p> | + | <p style="text-align:center;">Figure-6. Fluorescence change of single cell</p> |
</div> | </div> | ||
<p>Cells were diluted with culture medium and immobilized with glycerol. (5ul bacteria +20ulmedium+20ul glycerol). Making sure that the cell was alive and motionless, we could take photo of the same cell. From the pictures, we can see that the fluorescence changed with time in an oscillatory way, which supports our bio-oscillator design.</p> | <p>Cells were diluted with culture medium and immobilized with glycerol. (5ul bacteria +20ulmedium+20ul glycerol). Making sure that the cell was alive and motionless, we could take photo of the same cell. From the pictures, we can see that the fluorescence changed with time in an oscillatory way, which supports our bio-oscillator design.</p> | ||
<div><p><img src="https://static.igem.org/mediawiki/2013/0/01/HUST-proj-res7.jpg" /></p> | <div><p><img src="https://static.igem.org/mediawiki/2013/0/01/HUST-proj-res7.jpg" /></p> | ||
| - | <p>Figure-7. Fluorescence change of multicell</p> | + | <p style="text-align:center;">Figure-7. Fluorescence change of multicell</p> |
</div> | </div> | ||
<p>We used fluorospectro photometer to measure the oscillating behavior of multi-cells. | <p>We used fluorospectro photometer to measure the oscillating behavior of multi-cells. | ||
| Line 157: | Line 157: | ||
1.Replace the report gene mRFP by ygfD in the dual-feedback circuit to see whether the propionate generation can oscillate.<br> | 1.Replace the report gene mRFP by ygfD in the dual-feedback circuit to see whether the propionate generation can oscillate.<br> | ||
2.Regulate the period of propionate generation to cope with human’s BP rhythm. We may utilize the frequency divider with an ssrA-tag analog attached to the end of enzyme to achieve it.<br> | 2.Regulate the period of propionate generation to cope with human’s BP rhythm. We may utilize the frequency divider with an ssrA-tag analog attached to the end of enzyme to achieve it.<br> | ||
| - | <div style="float:right"><img src="https://static.igem.org/mediawiki/2013/d/d6/HUST-proj-future-work.png | + | <div style="float:right"><img src="https://static.igem.org/mediawiki/2013/d/d6/HUST-proj-future-work.png" width="200" /></div> |
3.Transform the regulatory net into bifidobacterium – microorganism has reputation among the dairy industry — due to people who we sent questionnaire to show preference eating food containing probiotics rather than E.coli, Also, we will measure the propionate outside of the human body.<br> | 3.Transform the regulatory net into bifidobacterium – microorganism has reputation among the dairy industry — due to people who we sent questionnaire to show preference eating food containing probiotics rather than E.coli, Also, we will measure the propionate outside of the human body.<br> | ||
Revision as of 07:24, 28 October 2013
Overview
Hypertension is an important worldwide public-health challenge.It has been identified as the leading risk factor for mortality mainly because it can lead to adverse cardiovascular events. It has already affected one billion people through out world and killed nine million people each year. And the incidence of it appears to follow a circadian pattern, reaching a peak in the morning shortly after wakening and arising.
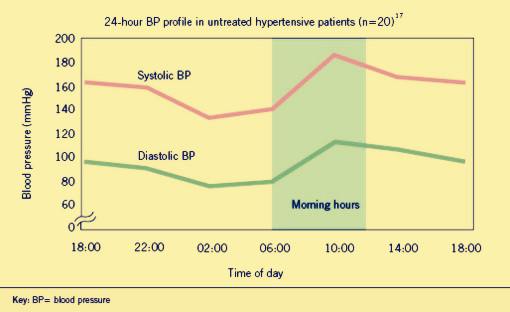
There is a wide selection of antihypertensive drugs designed for patients. However, traditional treatment comes along with heavy financial burden and severe side effects along with drug dependence. To make the matter worse, these medicines can not stop morning surge from taking patients' lives.
Short chain fatty acid(SCFA) ,especially acetate and propionate, was newly proved to cause an acute hypotensive response. A GPCRs called olfr78 expressed in smooth muscle cells of small blood vessel plays an important role. It could be activated by SCFA and induce vasodilatation and hypotension. In consideration of the case, we developed a novel method to treat hypertension by utilizing a group of friendly engineering bacteria which can release propionate periodically in human intestine.
To achieve this,we found a four-enzyme pathway in E.coil that converts succinate to propionate. By combining key genes with bio-oscillator, we try to make E.coli release propionate in patients’intestine periodically. Once the E.coli is delivered into human body as probiotics, the propionate can be taken by the circulatory system and act with the receptors. In that case, patients will not worry about excessive surge anymore.
Results
As we divided our project to two main parts: propionate generation and oscillator. The results will be demonstrated from these two sections.
Propionate generation:
1.We firstly successfully copied the four genes ygfD/ygfH/ygfG/Sbm from E.coli strain K12, and then recombined them independently to expression vector. Purified protein from cell disruptions by Ni-chelating and did gel analysis. From figure-1 , we confirmed that the enzymes were successfully expressed.

Figure1. SDS-PAGE analysis of Sbm, ygfD, ygfG, ygfH over-expression
2.As we thought the overexpression of enzymes didn’t directly prove the increase of propionate, and we wanted to find the key gene in the reaction. So we used the positive clones to do fermentation. We firstly optimized the condition and found the best sampling time points. Then we used standard propionate of seven gradients to draw a linear graph between propionate concentration and HPLC peak area. Figure-2 shows the relationship.

Figure-2. Standard curve of propionate and HPLC peak area
Later, we used the recombinant strain to do fermentation, and measured propionate concentration in the samples. Figure-3 shows the propionate increase percent of each gene recombinant strain. From the data, we found propionate production had a significant increase when ygfD transformed. In other words, we found the key gene- our oscillator’s output.

Figure-3. HPLC analysis wild-type BL21 and recombination BL21 with four genes
Oscillator:
1.As for the oscillator, we first successfully constructed the two plasmids formed the dual-feedback circuit. (Circuit and plasmid were show in figure-4) LAA tag was added to the protein for rapid degradation. Gene sequencing confirmed the plasmids to be correct without any lethal mutation.

Figure-4.The dual-feedback circuit and two plasmids we constructed
2.After that, we transformed recombinant plasmid pET-28a (+) which had an mRFP reporter to check if it could function well. We induced the positive clone with 2mM IPTG and observed by fluorescence microscope. Figure-5 shows that it function well.

Figure-5. Fluorescence microscope photo of recombinant plasmid pET-28a (+) transformed cell induced by 2mM IPTG
3.Later, we co-transformed two recombinant plasmids pET-28a (+) and pACYCDuet-1. After inducing positive clone by 0.7% Arabinose and 2 mM IPTG, we used fluorescence microscope to see RFP change of single cell and used fluorospectro photometer to see RFP change of multicells.Figure-6 & Figure-7 shows the result.

Figure-6. Fluorescence change of single cell
Cells were diluted with culture medium and immobilized with glycerol. (5ul bacteria +20ulmedium+20ul glycerol). Making sure that the cell was alive and motionless, we could take photo of the same cell. From the pictures, we can see that the fluorescence changed with time in an oscillatory way, which supports our bio-oscillator design.

Figure-7. Fluorescence change of multicell
We used fluorospectro photometer to measure the oscillating behavior of multi-cells. After induced by 0.7% arabinose and 2 mM IPTG when OD600 was 0.55, cells were cultured in 37℃/200rpm.We sampled a series of time points and draw the curve as figure-7. We saw a significant fluorescence oscillating in compare to control group. The control group cells transformed pET-28a (+) only.
Summarization: We have successfully found a way to enhance cells generation of propionate, tested and verified our design of bio-oscillator. Combining these two works, we believe we can build a gut probiotic which can release propionate periodically in accord with the rhythm of human BP.
Future work
We do not have enough time to fulfill the whole project. Based on the pre-existing work, these tasks are coming to be finished in future.
1.Replace the report gene mRFP by ygfD in the dual-feedback circuit to see whether the propionate generation can oscillate.
2.Regulate the period of propionate generation to cope with human’s BP rhythm. We may utilize the frequency divider with an ssrA-tag analog attached to the end of enzyme to achieve it.

Judging Critieria
Already registered in the official website in 13th March and was accepted in 12th April.
1.We completed safety form, judging form and team wiki before the deadline. It is for sure that we are going to present a poster and a talk at the iGEM Jamboree.
2.We documented four newly standard BioBrick Part(sbm/ygfG/ygfH/ygfD) used in our project and submitted them to the iGEM Registry adhere to guidelines.
3.Our works aims at maintaining the blood pressure through microbe metabolism SCFA, which is a new application in medicine to our knowledge.
4.We did plenty of experiment to validate that two of BioBrick Part of our own design and construction works as expected.
5.We share information and material with WHU and HZAU .Cooperating with HZAU on characterizing one part.
6.We originaly creat a crossword to popularize historical knowledge about iGEM. That's a good new approach for human practice.
Therefore, we believe that we deserve a Gold Medal Prize.
 "
"









