|
|
| (4 intermediate revisions not shown) |
| Line 72: |
Line 72: |
| | position:relative; | | position:relative; |
| | width:1100px; | | width:1100px; |
| - | height:1600px; | + | height:1900px; |
| | z-index:1; | | z-index:1; |
| | background-color: #FFF; | | background-color: #FFF; |
| Line 79: |
Line 79: |
| | position:relative; | | position:relative; |
| | width:1025px; | | width:1025px; |
| - | height:1600px; | + | height:1900px; |
| | z-index:1; | | z-index:1; |
| | background-color: #FFF; | | background-color: #FFF; |
| Line 132: |
Line 132: |
| | </div> | | </div> |
| | <div id="partable"> | | <div id="partable"> |
| - | | + | <p2> |
| - | <p2>
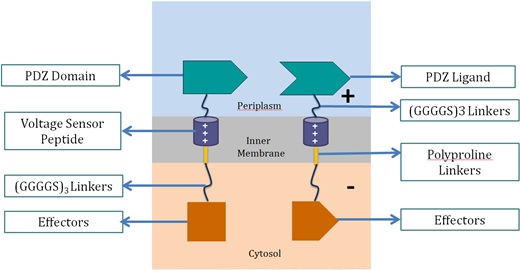
| + | <p>Voltage Switch is a response system triggered by a change of potential across the bacterial inner membrane. Components of this system include the transmembrane voltage sensor peptide originated from potassium ion channels, polyproline linker in the inner membrane, glycine serine linker, PDZ domain and PDZ ligand in the periplasm, and glycine serine linker and effectors in the cytosol.</p> |
| - | <h1>Background</h1>
| + | <h3>1. Components of Voltage Switch System</h3><p align="center"> |
| - | <p>The voltage-dependent ion channels family are a group of ion channel proteins found to have permeability to ions dependent on the voltage in the external environment. This family of ion channels are widely found in neuronal cells, which transmit signal through electricity pulse. It was found that inside the ion channels, there exist a few repeating short peptides that are responsible for voltage sensing and the open and close of the channel. Studies have shown that this peptide can migrate across the membrane in responds to voltage, and analysis of the peptide showed numerous positively charged arginine and hydrophobic leucine. However, there were not much applications. In this project we will introduce this voltage sensor peptide as our novel switch, thus achieve high speed respond in bacteria either in form of biomolecular fluorescent complementation, or increased reaction rate in the degradation pathway of carcinogenic substance BaP.</p>
| + | <img src="https://static.igem.org/mediawiki/2013/8/84/Vs1.png" alt="2" width="521" height="270"> <br> |
| - | <h1>Project Description</h1>
| + | </p> |
| - | <h3>Voltage Switch</h3>
| + | <p><stong> |
| - | <p>Having inspired by the voltage sensor peptide, we decided to make use of it as our novel voltage switch. </p>
| + | <strong>Voltage Sensor Peptide</strong></strong><br> |
| - | <h3>PDZ Domain/Ligand</h3>
| + | Voltage sensor peptide, originated from the archaea <em>Aeropyrum pernix</em>, is a protein domain found in a voltage-dependent potassium ion channel. Previous studies suggested that the domain could translocate within the membrane upon membrane potential change [10, 11]. This helix peptide received its voltage-sensing ability from its four positively-charged arginine residues, and high hydrophobicity from its numerous leucine residues.</p> |
| - | <p>We made use of the PDZ domain and ligand found in <em>Mus musculus</em> as a pair of dimers that link two voltage sensor peptide together. As demonstrated by the SJTU iGEM team of 2012, the PDZ domain and ligand functioned and worked as a membrane protein scaffolding part which links enzymes together through linkers. Since both voltage sensor peptides are positively charged, without the help of the PDZ domain and ligand, they would not bind together due to mutual repulsion. With the PDZ domain and ligand, the 2 voltage sensor peptides would dimerize, thus forming the complete voltage switch.</p>
| + | <p><strong>PDZ Domain and PDZ Ligand</strong><br> |
| - | <h3>Voltage Sensor Peptide</h3>
| + | This is a dimer found in <em>Mus musculus</em>, and were used by the 2012 SJTU iGEM team as a membrane scaffolding tool. The PDZ dimer provides the necessary linkage of the two fusion proteins in the voltage switch system.</p> |
| - | <p>
| + | <p><strong>Polyproline Linker</strong><br> |
| - | Found in <em>Aeropyrum pernix</em>, the voltage sensor peptide contains arginine residues along the peptide, which give the peptide a net positive charge under phyiological pH. It also contains a lot of leucine and other hydrophobic residues, which make it an excellent transmembrane domain. The voltage sensor peptide itself is very short. We used a polyproline rigid linker to elongate it so that the separation distance would be longer. In order to link the part with the PDZ domain and ligand, and also the downstream effectors, we added in a polyglycine linker in front and after the whole part. The rotatable angles on glycine residues allow flexible movement of the whole part.</p>
| + | Polyproline linker is a pure proline rigid rod. Its rigidity is due to the limited rotation of peptide bonds in proline, a ring-shaped amino acid. Also, proline does not contain any hydrophilic functional group, which makes the linker hydrophobic and thus able to stay within the inner membrane.</p> |
| - | <h3>Effectors</h3>
| + | <p><strong>Glycine Serine Linker</strong><br> |
| - | <p>We made use of Biomolecular fluorescent complementation and 2 enzymes to demonstrate how the voltage sensors worked.</p>
| + | This is a hydrophilic flexible linker which links PDZ dimer with voltage sensor peptides. It also links the polyproline linkers in the membrane with the effectors in the cytosol.</p> |
| - | <p>Biomoleular fluorescent complementation (BiFC)</p>
| + | <p><strong>Effectors</strong><br> |
| - | <p>BiFC is a phenomenon found in fluorescent proteins that, if we cleaved the protein at some specific sites in the loops, we can separate the protein into 2 parts, the N-terminal and the C-terminal. These two parts are non-fluorescence, and under normal physiological condition, they will not refold with the other parts to give fluorescent. However, when there is protein-protein interactions in which the interacting proteins are linking with the fragments, the interactions can bring the two fragments together, where they refold and mature to give fluorescent again. We make use of this feature to be our reporter. When the switch is OFF, the fragments were far apart, so they don’t fluoresce, but when we turn ON the switch, it brings the fragments together, thus the fragments refold to give fluoresence. One drawback of this system is that there were still no reported reversible BiFC reaction, and so theoretically it can’t be switched off.</p>
| + | These effectors can be changed accordingly. In our project, we chose laccase and dioxygenase for BaP degradation. We also planned to test the system with bimolecular fluorescence complementation (BiFC) dimerization. |
| - | <h3>BaP Degradation</h3><p>
| + | To become functional, the PDZ domain and PDZ ligand bind the two protein fragments together, forming a stable dimer.</p> |
| - | Laccase and Dioxygenase are 2 enzymes found in different organisms, which can participate in a pathway in which carcinogenic substances BaP is first degraded into quinone, and then to some simple carboxylic acids that could either be harmless or very little harmful. Normally, the enzymatic reaction rate in cells won’t be very high regardless of the enzyme activity, and this is because in physiological conditions and most experimental setups, rate of diffusion of substrates is the major limiting factor. We try to make use of our voltage switch as a way to quickly alter the distance between enzymes to change the substrate diffusion distance, thus the reaction rate can be controlled by electricity.</p>
| + | <p><h3>2. Proposed Mechanism of Switching “ON” and “OFF” in the System</h3></p> |
| - | <h3>How It Works</h3>
| + | <p><strong>Switching “OFF”</strong><br> |
| - | <p>The voltage switch is a novel protein switch that responds to external voltage. The switch itself consist of the PDZ Ligand-Voltage sensor peptide (<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1092007">BBa_K1092007</a>) and the PDZ Domain-Voltage sensor peptide (<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1092008">BBa_K1092008</a>), and can be linked to different effectors such as the Dioxygenase (<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1092002">BBa_K1092002</a>) and Laccase (<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1092004">BBa_K1092004</a>), or the RFP fragments (<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1092105">BBa_K1092105</a> & <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1092106">BBa_K1092106</a>). Initially, the two proteins would express and localize onto the inner membrane of the bacteria. The two peptides would then come together forming a dimer. After that, due to the mutual repulsion of the positive charges in the two voltage sensor peptide, the two voltage sensor peptide would separate (Fig. 1). This separate the two effectors down below the two peptide, causing either a long distance for the two effectors to interact, or a longer distance of diffusion of substrates. This represent the OFF stage of the voltage switch, and it could be enhanced by using negatively charged environment to further pull them apart.<br />
| + | When no external charge is applied, the two effectors are combined, as the repulsion force between the two positively-charged voltage sensor peptide is relatively smaller than the dimer effect. The two voltage sensor peptides are vertically parallel to each other.</p> |
| - | <br />
| + | <p align="center"><img src="https://static.igem.org/mediawiki/2013/0/0f/Vs2.png" alt="3" width="472" height="288"></p> |
| - | During the ON stage, the environment become positively charged, and this environment exert a strong electrostatic repulsion on the two voltage sensor peptide so that they can be pushed together by overcoming the mutual repulsion between the peptides (Fig. 2). This action brings the two downstream effectors together, which either provides short-enough distance for them to interact, or greatly reduces the distance of diffusion of the substrates, thus greatly enhance the reaction rate.</p>
| + | <p><strong>Switching “ON”</strong><br> |
| - | <p> </p> | + | When negative charge is applied into the system, the two effectors are separated. As the potential across the membrane is inverted, there is an attractive force between negatively-charged periplasm and positively-charged voltage sensor peptide, and vice versa for cytosol and voltage sensor peptide. Together, these two forces pull the two voltage sensor peptides from vertical position to more horizontal, which causes the separation of the effectors.</p> |
| | + | <p align="center"><img src="https://static.igem.org/mediawiki/2013/d/d3/Vs3.png" alt="4" width="482" height="292"><br clear="all"> |
| | + | </p> |
| | + | |
| | </p2> | | </p2> |
| | </div> | | </div> |
 "
"