Team:DTU-Denmark/Notebook/6 August 2013
From 2013.igem.org
(→Gel 3) |
(→Procedure) |
||
| Line 33: | Line 33: | ||
===PCR to extract Nir2 from P. aeruginosa=== | ===PCR to extract Nir2 from P. aeruginosa=== | ||
| + | ===PCR for araBAD and SPL with DMSO=== | ||
| + | Template and primers as previous. | ||
| + | ===PCR on Nir1 with MgCl2 gradient=== | ||
| + | Used previous isolation from a gel purification to make PCR for amplification of the strand. Used 5% DMSO as additive. The PCR was done with primer pair 41 and with x7 polymerase at 55C annealing and 5:00 min extension. Also we used 20 sec as denaturing time instead of the normal 10. | ||
| + | The magnesium gradient was as follow: | ||
| + | *0 | ||
| + | *1uL MgCl2 2mM per 50uL reaction | ||
| + | *5uL MgCl2 2mM per 50uL reaction | ||
| + | *20uL MgCl2 2mM per 50uL reaction | ||
| - | === | + | ===Gel purification=== |
of the Nir1 fragment extraced from P. aeruginosa. Nanodrop: 8ng/uL | of the Nir1 fragment extraced from P. aeruginosa. Nanodrop: 8ng/uL | ||
| - | === | + | ===Plasmid miniprep=== |
HAO in pZA21 from USER cloning was purified for restriction analysis. Nanodrop measurement: 180ng/uL | HAO in pZA21 from USER cloning was purified for restriction analysis. Nanodrop measurement: 180ng/uL | ||
Revision as of 07:41, 7 August 2013
6 August 2013
Contents |
lab 208
Main purpose
Who was in the lab
Kristian, Gosia, Natalia, Henrike
Procedure
PCR for AMO
Made four reactions using 1 uL respectively 10 uL of culture from the -20 or from the glycerol stock (-80) as template and adjusted volume of water accordingly.
Primers 10a, 10b.
PCR for SPL (synthtic promoter library)
template: pZa21 with RFP, primers: 52a, 52b1, temperature: 50C, time: 2:30
PCR for reference promoter
template: pZa21 with RFP, primers: 52a, 52b2, temperature: 50C, time: 2:30
PCR for araBAD K808000
template: K808000, primers: 12a, 12bn, temperature: 50C, time: 2:30
PCR to extract Nir2 from P. aeruginosa
PCR for araBAD and SPL with DMSO
Template and primers as previous.
PCR on Nir1 with MgCl2 gradient
Used previous isolation from a gel purification to make PCR for amplification of the strand. Used 5% DMSO as additive. The PCR was done with primer pair 41 and with x7 polymerase at 55C annealing and 5:00 min extension. Also we used 20 sec as denaturing time instead of the normal 10. The magnesium gradient was as follow:
- 0
- 1uL MgCl2 2mM per 50uL reaction
- 5uL MgCl2 2mM per 50uL reaction
- 20uL MgCl2 2mM per 50uL reaction
Gel purification
of the Nir1 fragment extraced from P. aeruginosa. Nanodrop: 8ng/uL
Plasmid miniprep
HAO in pZA21 from USER cloning was purified for restriction analysis. Nanodrop measurement: 180ng/uL
Results
Gel 1
1% agarose gel
- 1 kb ladder
- Nir1 col. T
- Nir1 col. T
- Nir2 col. T
- Nir2 col. T
- Nir1 frag. T
- Nir1 frag. T
- Nir1 col. X7
- Nir1 col. X7
- neg
- Hi
- Hi
- DMSO
- DMSO
- Nir1
- Nir1
- Nir2
- Nir2
- 1 kb ladder
Gel 2
1% agarose gel
- 1 kb ladder
- NirW
- NirW
- araBAD biobrick K808000
- araBAD biobrick K808000
- SPL in pZA21 containing RFP
- SPL in pZA21 containing RFP
- reference promoter in pZA21 containing RFP
- reference promoter in pZA21 containing RFP
- neg
- neg
- 1 kb ladder
Gel 3
1% agarose gel
- 1 kb ladder
- AMO, 1uL of -20 culture as template
- AMO, 10uL of -20 culture as template
- AMO, 1uL of glycerol stock as template
- AMO, 10uL of glycerol stock as template
- neg from AMO PCR
- 1
- 2
- 3
- 4
- 5
- 6
- 1 kb ladder
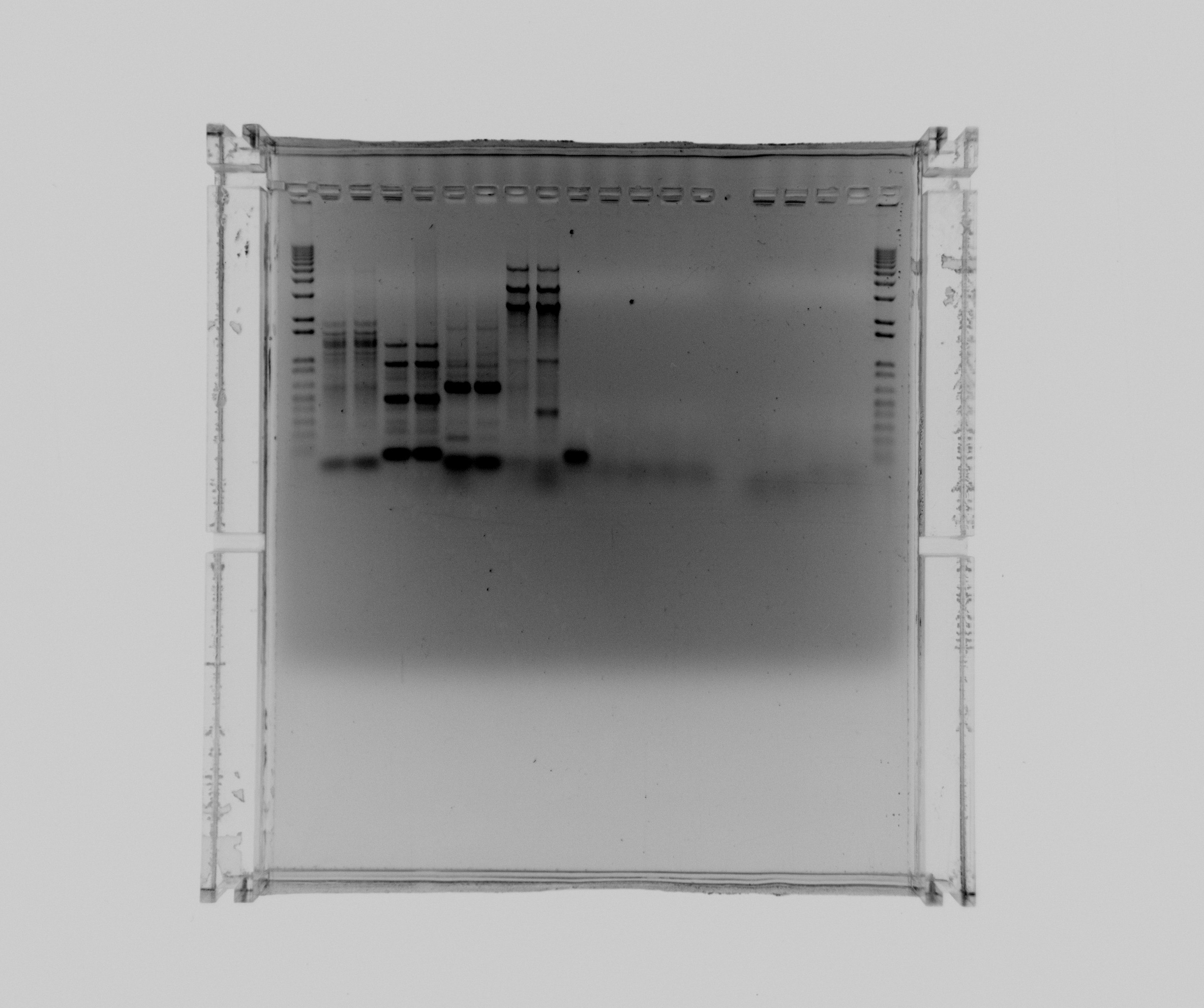
Gel result:
- 1 - ladder
- 2 - sample nr 1 - SPL, primers 52a, 52b1, template pZA21 with RFP, 50C, 2:30 min extension
- 3 - sample nr 2 - SPL, primers 52a, 52b1, template pZA21 with RFP, 50C, 2:30 min extension
- 4 - sample nr 3 - ref, primers 52a, 52b2, template pZA21 with RFP, 50C, 2:30 min extension
- 5 - sample nr 4 - ref, primers 52a, 52b2, template pZA21 with RFP, 50C, 2:30 min extension
- 6 - sample nr 5 - araBAD, primers 12a, 12bn, template K80800, 50C, 2:30 min extension
- 7 - sample nr 6 - araBAD, primers 12a, 12bn, template K80800, 50C, 2:30 min extension
- 8 - sample nr 7 - Nir2, extraction PCR, primers 42a, 42b, cells in water, Phusion polymerase
- 9 - sample nr 8 - Nir2, extraction PCR, primers 42a, 42b, cells in water, Phusion polymerase
- 10 - sample nr 9 - Nir2, extraction PCR, primers 42a, 42b, cells in water, x7 polymerase
- 11 - sample nr 10 - Nir2, extraction PCR, primers 42a, 42b, cells in water, x7 polymerase
- 12 - sample nr 11 - Nir2, extraction PCR, primers 42a, 42b, cells in water, plus MgCl2 additive, Phusion polymerase
- 13 - sample nr 12 - - Nir2, extraction PCR, primers 42a, 42b, cells in water, plus MgCl2 additive, x7 polymerase
- 14 - sample nr 13 - Nir1 with USER primers, primers 39a, 39b, x7, template - Nir purified from gel
- 15 - sample nr 14 - Nir1 with USER primers, primers 39a, 39b, x7, template - Nir purified from gel
- 16 - sample nr 15 - check Nir1 with primers for NirM+S, primers 11a, 44, template - Nir purified from gel, Phusion polymerase
- 17 - sample nr 16 - check Nir1 with primers for NirM+S, primers 11a, 44, template - Nir purified from gel, Phusion polymerase
- 18 - ladder
Conclusion
Navigate to the Previous or the Next Entry
 "
"