Team:BYU Provo/Notebook/SmallPhage/Springexp/Period2/PR
From 2013.igem.org
(Difference between revisions)
| Line 67: | Line 67: | ||
5.20 T7 Minor Capsid Protein PCR | 5.20 T7 Minor Capsid Protein PCR | ||
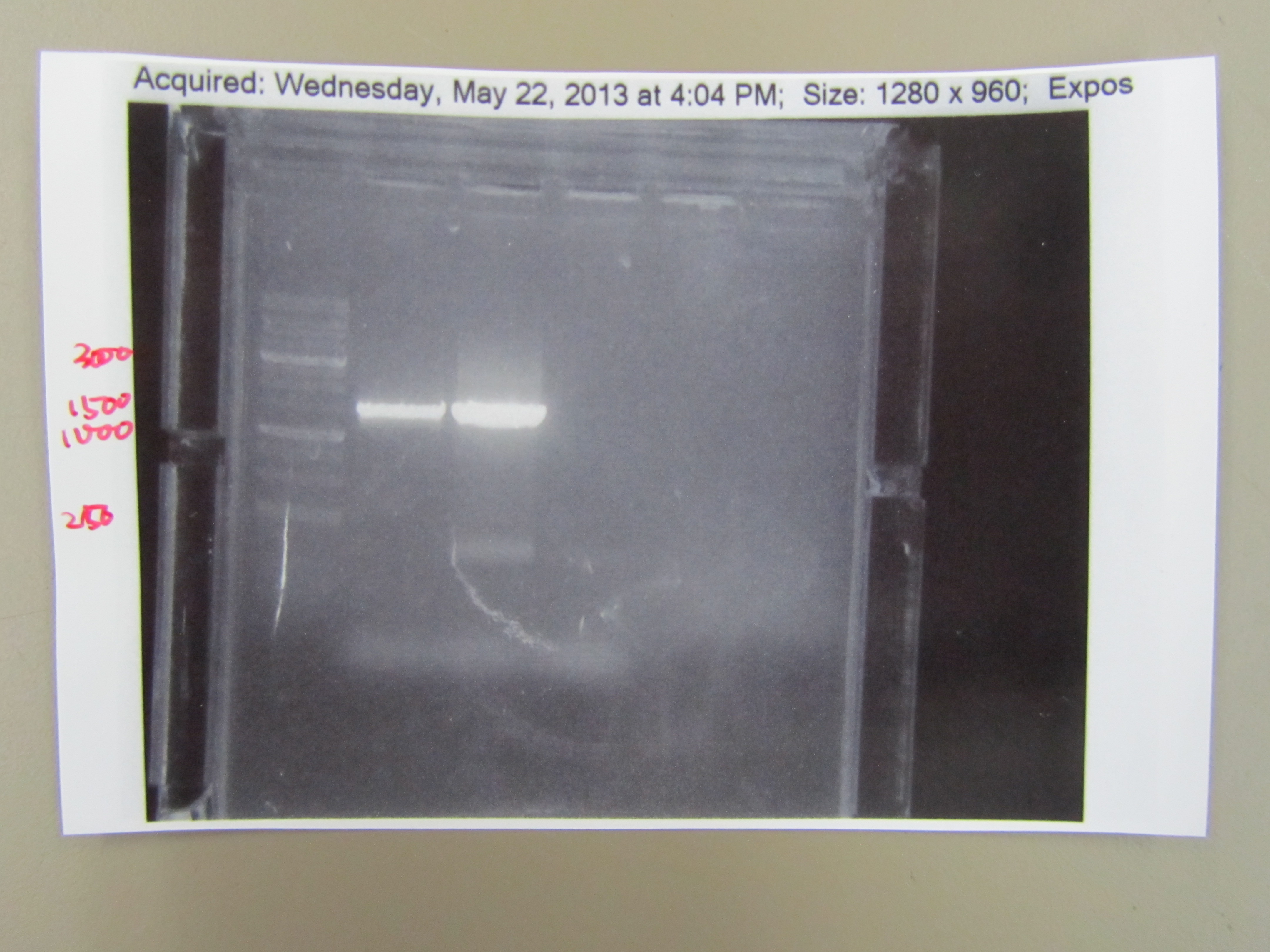
| - | : In order to sequence the T7 minor capsid protein, we had to amplify the gene using PCR. We first isolated the DNA by boiling phage in a PCR machine and centrifuging it for a minute, leaving the T7 DNA in the supernatant. Then we added ddH20, TAQ buffer, dNTPs, the forward and reverse minor capsid protein primers, the template DNA from the supernatant, and TAQ polymerase in a PCR tube. We ran 35 cycles of PCR and then froze it. To make sure we isolated the T7 minor capsid protein, we ran it with gel electrophoresis and found a single band that corresponds with the the proper base pair length. This showed that we successfully | + | : In order to sequence the T7 minor capsid protein, we had to amplify the gene using PCR. We first isolated the DNA by boiling phage in a PCR machine and centrifuging it for a minute, leaving the T7 DNA in the supernatant. Then we added ddH20, TAQ buffer, dNTPs, the forward and reverse minor capsid protein primers, the template DNA from the supernatant, and TAQ polymerase in a PCR tube. We ran 35 cycles of PCR and then froze it. To make sure we isolated the T7 minor capsid protein, we ran it with gel electrophoresis and found a single band that corresponds with the the proper base pair length. This showed that we successfully cloned the T7 minor capsid protein gene. |
[[File:5.22PCRgel.JPG|400px|center]] | [[File:5.22PCRgel.JPG|400px|center]] | ||
Revision as of 01:41, 29 May 2013
| Small Phage Spring Notebook: May 13 - May 26 Progress Report
| ||
|
|
1. Goals for the week
5.9 T7+ Liquid Culture Phage Concentration Test #2
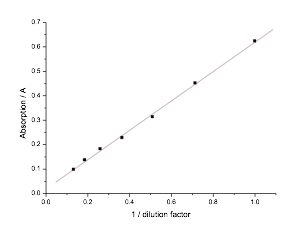
5.13 Determining E. coli Concentration With Spectrophotometer
5.15 Titer Test on 5.3 T7 new Phage Stock
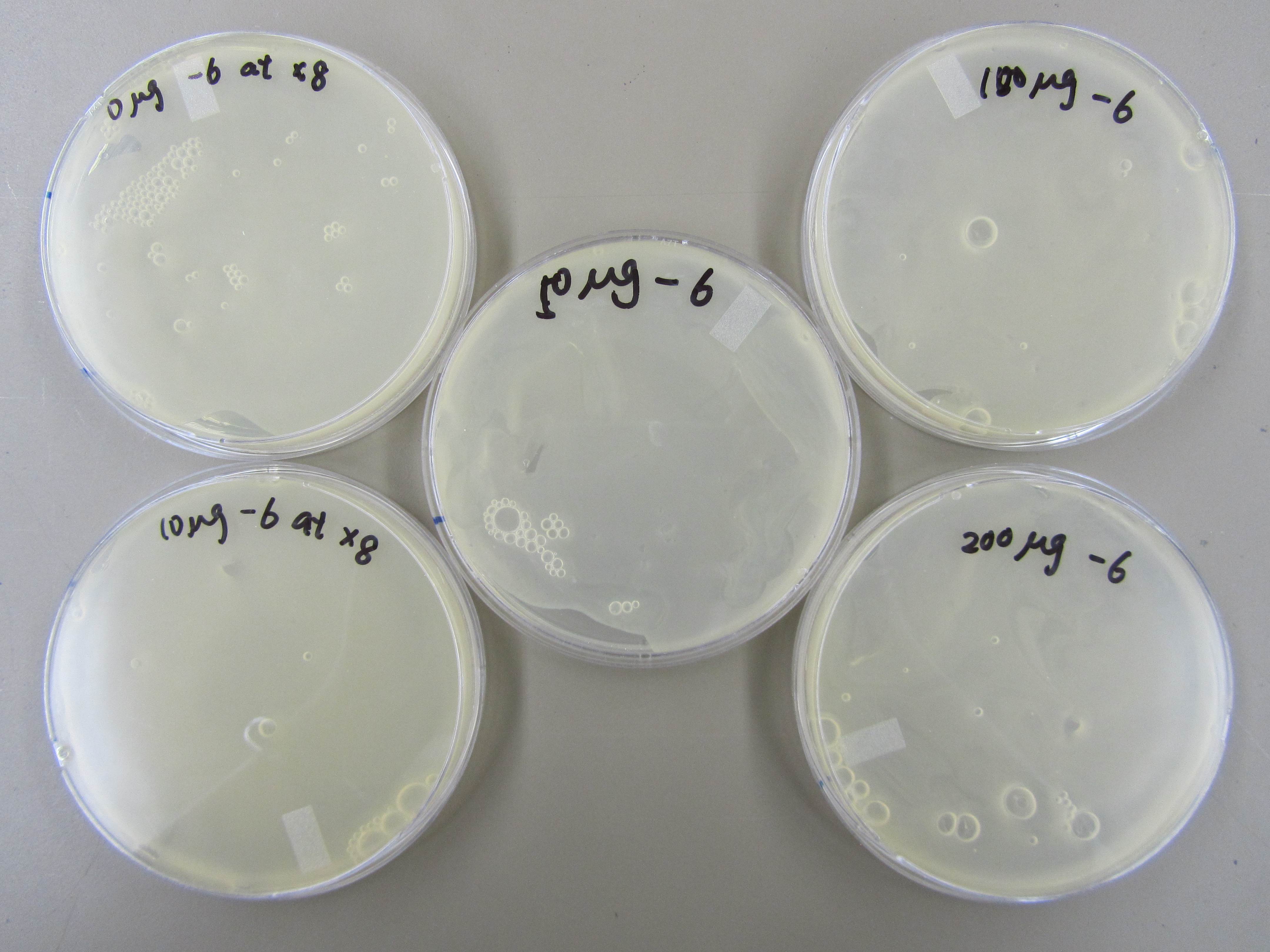
5.20 Mutagen Concentration Experiment
5.20 T7 Minor Capsid Protein PCR
| |
 "
"