Team:Yale/Project MAGE
From 2013.igem.org
(Difference between revisions)
(→MAGE Targets) |
(→MAGE Targets) |
||
| Line 28: | Line 28: | ||
**However, we used the literature available to locate spots where we would want to introduce mutations | **However, we used the literature available to locate spots where we would want to introduce mutations | ||
| + | |||
| + | {| | ||
| + | |- | ||
| + | | | ||
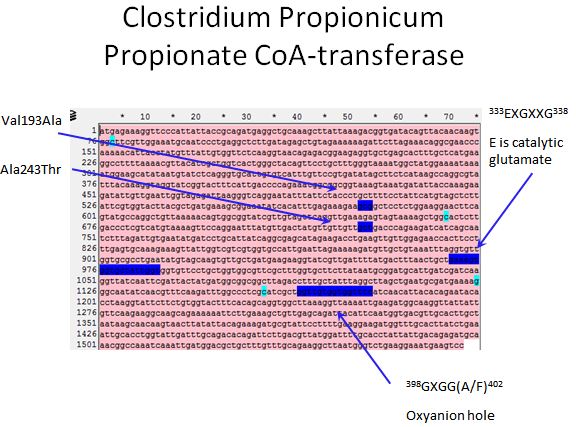
==== Propionate CoA-transferase ==== | ==== Propionate CoA-transferase ==== | ||
*According to Selmer et al. 2002, there is a catalytic glutamate found in all CoA-transferases | *According to Selmer et al. 2002, there is a catalytic glutamate found in all CoA-transferases | ||
| Line 33: | Line 37: | ||
**Also, we were able to locate the oxyanion hole of the enzyme | **Also, we were able to locate the oxyanion hole of the enzyme | ||
**These two regions along with those mentioned in the Lee paper's were the targets for our MAGE oligos (4 in total) | **These two regions along with those mentioned in the Lee paper's were the targets for our MAGE oligos (4 in total) | ||
| - | [[File:PCTmagetargets.jpg]] | + | |style="padding-left: 20px; padding-right: 20px;"|[[File:PCTmagetargets.jpg|400px]] |
| + | |} | ||
| + | |||
| + | |||
| + | {| | ||
| + | |- | ||
| + | | | ||
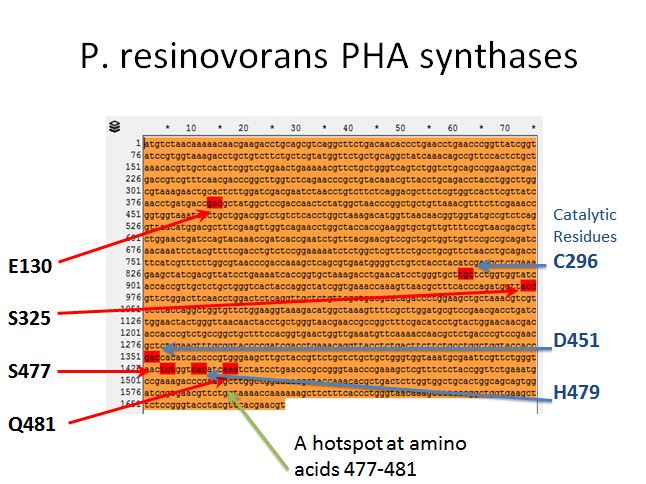
| + | ==== P. resinovorans PHA synthases ==== | ||
| + | *From Yang et al. 2011 we knew there were 4 targets for mutation that affected the efficiency of this enzyme (E130, S325, S477, and Q481) | ||
| + | *After some more reading we found three amino acids that were putative catalytic residues (C296, D451, and H479) | ||
| + | *This gave the impression of one hotspot area (amino acids 477-481) so an oligo was designed to target the enzymes in between (AA478 and AA480) | ||
| + | |||
| + | |style="padding-left: 20px; padding-right: 20px;"|[[File:PHAMAGE.JPG|400px]] | ||
| + | |} | ||
| + | |||
=== Pathway Engineering === | === Pathway Engineering === | ||
Revision as of 19:03, 31 August 2013
| Project Overview | Validate PLA synthesis | Develop bioassay | Apply MAGE | Introduce export system | Make a bioplastic |
|---|
Contents |
Aims for the Project
- Engineer strains of E. coli to validate PLA synthesis
- Develop bioassay to screen PLA production
- Apply MAGE to optimize PLA production, guided by FBA
- Introduce type 1 secretion system to export and extract PLA
- Make a bioplastic
MAGE Targets
- The first step in applying MAGE is finding MAGE targets. This involved reading numerous scientific papers learning as much as possible about the heterologous enzymes, and the pathway that was being used to create the PLA
Enzyme Targets
- Sadly there was no crystal structure of either enzyme we could use to locate the sites to introduce mutations
- However, we used the literature available to locate spots where we would want to introduce mutations
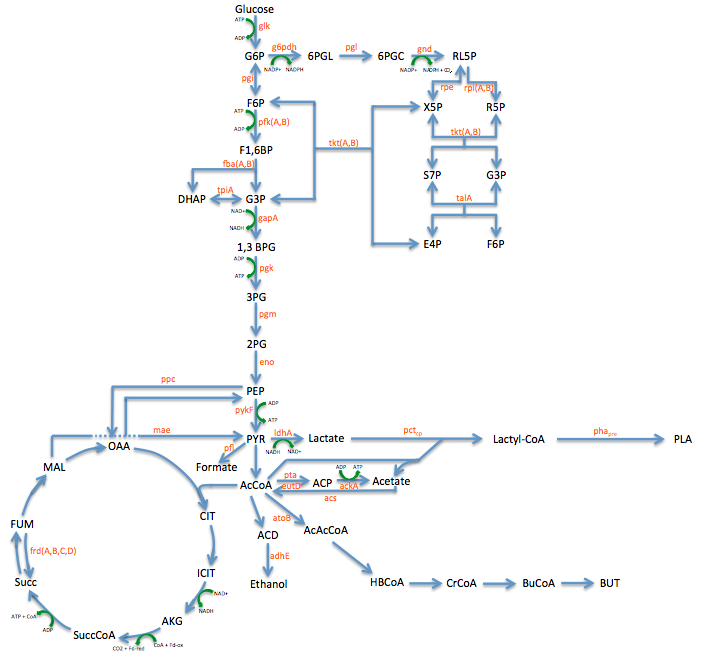
Pathway Engineering

List of Papers:
Jacob et al. 1997
Matsuzaki et al. 1998
Sawers et al. 1998
Park et al. 2002
Selmer et al. 2002
Takase et al. 2002
Fong et al. 2005
Matsumoto et al. 2005
Rangarajan ES et al. 2005
Matsumoto et al. 2006
Jung et al. 2009
Matsumoto et al. 2009
Juang et al. 2010
Orth et al. 2010
Yang et al. 2011
Kandasamy et al. 2012
Yang et al. 2013
 "
"