Template:Kyoto/Notebook/Aug 23
From 2013.igem.org
(Difference between revisions)
(→Gel Extraction) |
(→Gel Extraction) |
||
| (23 intermediate revisions not shown) | |||
| Line 40: | Line 40: | ||
|17||Fusion1 attenuator||--||-- | |17||Fusion1 attenuator||--||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku_Aug23electrophoresis.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
| Line 78: | Line 78: | ||
|17||tetR aptamer 12_P||--||-- | |17||tetR aptamer 12_P||--||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku_Aug23electrophoresis2.jpg]]<br> |
<span class="author">Kojima and Honda</span> | <span class="author">Kojima and Honda</span> | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 117: | Line 117: | ||
|17||tetR aptamer 12_1R||--||-- | |17||tetR aptamer 12_1R||--||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug23 Electrophoresis(N2).jpg]]<br> |
</div> | </div> | ||
| Line 124: | Line 124: | ||
<span class="author">Nakamoto and Kojima</span> | <span class="author">Nakamoto and Kojima</span> | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !Fusion6 antisense (1)||EcoRI||SpeI||buffer B||BSA||MilliQ||total | + | ! ||Fusion6 antisense (1)||EcoRI||SpeI||buffer B||BSA||MilliQ||total |
|- | |- | ||
| - | |3||0.5||0.5||3||0.3||22.7||30 | + | |2 cuts||3||0.5||0.5||3||0.3||22.7||30 |
|- | |- | ||
| - | |0.6||0.1||0||1||0.1||8.2||10 | + | |1 cut||0.6||0.1||0||1||0.1||8.2||10 |
|- | |- | ||
| - | |0.6||0||0.1||1||0.1||8.2||10 | + | |1 cut||0.6||0||0.1||1||0.1||8.2||10 |
|- | |- | ||
| - | |0.6||0||0||1||0.1||8.3||10 | + | |NC||0.6||0||0||1||0.1||8.3||10 |
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !Fusion6 antisense (1)||XbaI||PstI||buffer D||BSA||MilliQ||total | + | ! ||Fusion6 antisense (1)||XbaI||PstI||buffer D||BSA||MilliQ||total |
|- | |- | ||
| - | |3||0.5||0.5||3||0.3||22.7||30 | + | |2 cuts||3||0.5||0.5||3||0.3||22.7||30 |
|- | |- | ||
| - | |0.6||0.1||0||1||0.1||8.2||10 | + | |1 cut||0.6||0.1||0||1||0.1||8.2||10 |
|- | |- | ||
| - | |0.6||0||0.1||1||0.1||8.2||10 | + | |1 cut||0.6||0||0.1||1||0.1||8.2||10 |
|- | |- | ||
| - | |0.6||0||0||1||0.1||8.3||10 | + | |NC||0.6||0||0||1||0.1||8.3||10 |
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !Fusion1 antisense (1)||EcoRI||SpeI||buffer B||BSA||MilliQ||total | + | ! ||Fusion1 antisense (1)||EcoRI||SpeI||buffer B||BSA||MilliQ||total |
|- | |- | ||
| - | |3.3||0.5||0.5||3||0.3||22.4||30 | + | |2 cuts||3.3||0.5||0.5||3||0.3||22.4||30 |
|- | |- | ||
| - | |0.7||0.1||0||1||0.1||8.1||10 | + | |1 cut||0.7||0.1||0||1||0.1||8.1||10 |
|- | |- | ||
| - | |0.7||0||0.1||1||0.1||8.1||10 | + | |1 cut||0.7||0||0.1||1||0.1||8.1||10 |
|- | |- | ||
| - | |0.7||0||0||1||0.1||8.2||10 | + | |NC||0.7||0||0||1||0.1||8.2||10 |
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !pT181 antisense (1)||EcoRI||SpeI||buffer B||BSA||MilliQ||total | + | ! ||pT181 antisense (1)||EcoRI||SpeI||buffer B||BSA||MilliQ||total |
|- | |- | ||
| - | |5.6||0.5||0.5||3||0.3||20.1||30 | + | |2 cuts||5.6||0.5||0.5||3||0.3||20.1||30 |
|- | |- | ||
| - | |1.1||0.1||0||1||0.1||7.7||10 | + | |1 cut||1.1||0.1||0||1||0.1||7.7||10 |
|- | |- | ||
| - | |1.1||0||0.1||1||0.1||7.7||10 | + | |1 cut||1.1||0||0.1||1||0.1||7.7||10 |
|- | |- | ||
| - | |1.1||0||0||1||0.1||7.8||10 | + | |NC||1.1||0||0||1||0.1||7.8||10 |
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !pT181 antisense (1)||XbaI||PstI||buffer D||BSA||MilliQ||total | + | ! ||pT181 antisense (1)||XbaI||PstI||buffer D||BSA||MilliQ||total |
|- | |- | ||
| - | |5.6||0.5||0.5||3||0.3||20.1||30 | + | |2 cuts||5.6||0.5||0.5||3||0.3||20.1||30 |
|- | |- | ||
| - | |1.1||0.1||0||1||0.1||7.7||10 | + | |1 cut||1.1||0.1||0||1||0.1||7.7||10 |
|- | |- | ||
| - | |1.1||0||0.1||1||0.1||7.7||10 | + | |1 cut||1.1||0||0.1||1||0.1||7.7||10 |
|- | |- | ||
| - | |1.1||0||0||1||0.1||7.8||10 | + | |NC||1.1||0||0||1||0.1||7.8||10 |
|} | |} | ||
*incubate at 37°C from 17:29. | *incubate at 37°C from 17:29. | ||
| Line 221: | Line 221: | ||
|17||100bp||--||-- | |17||100bp||--||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug23 Electrophoresis (N3).jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
| Line 234: | Line 234: | ||
|- | |- | ||
|5||100bp||--||-- | |5||100bp||--||-- | ||
| + | |- | ||
| + | |6||J23100||SpeI||PstI | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug23 Electrophoresis(N3)-2.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
| Line 267: | Line 269: | ||
|16||100bp||--||-- | |16||100bp||--||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug23electrophoresis4.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
| Line 299: | Line 301: | ||
|16||100bp||--||-- | |16||100bp||--||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug23electrophoresis5.jpg]]<br> |
</div> | </div> | ||
| Line 327: | Line 329: | ||
|12 | |12 | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug23Gel Extraction.jpg]]<br> |
| - | [[File: | + | [[File:Igku Aug23 Gel Extraction(N6)-1.2.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Lane||DNA||Enzyme | !Lane||DNA||Enzyme | ||
| Line 350: | Line 352: | ||
|12 | |12 | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug23 Gel Extraction(N7)-2.jpg]]<br> |
| - | + | ||
{| class="wikitable" | {| class="wikitable" | ||
!Lane||DNA||Enzyme | !Lane||DNA||Enzyme | ||
| Line 361: | Line 363: | ||
|3 | |3 | ||
|- | |- | ||
| - | |5||rowspan=2| | + | |5||rowspan=2|aptamer 12_1M||rowspan=2|EcoRI & SpeI |
|- | |- | ||
|6 | |6 | ||
| Line 370: | Line 372: | ||
|1||100bp ladder||-- | |1||100bp ladder||-- | ||
|- | |- | ||
| - | |2||rowspan=2| | + | |2||rowspan=2|aptamer 12_P||rowspan=2|EcoRI & SpeI |
|- | |- | ||
|3 | |3 | ||
|- | |- | ||
| - | |5||rowspan=2| | + | |5||rowspan=2|aptamer 12_1R||rowspan=2|EcoRI & SpeI |
|- | |- | ||
|6 | |6 | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug23 Gel Extraction(N6)-2.4.jpg]]<br> |
| - | [[File: | + | [[File:Igku Aug23 Gel Extraction(N6)-3-2.jpg]]<br> |
<span class="author">Ashida</span> | <span class="author">Ashida</span> | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 402: | Line 404: | ||
|12 | |12 | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug23 Gel Extracton(N8)-1.jpg]]<br> |
| - | [[File: | + | [[File:Igku Aug23 Gel Extraction(N8)-1.2.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Lane||DNA||Enzyme | !Lane||DNA||Enzyme | ||
| Line 413: | Line 415: | ||
|4 | |4 | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug23 Gel Extraction(N7)-1.jpg]]<br> |
| - | [[File: | + | [[File:Igku Aug23 Gel Extraction(N7)1 2.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Lane||DNA||Enzyme | !Lane||DNA||Enzyme | ||
| Line 435: | Line 437: | ||
|6 | |6 | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug23 Gel Extraction(N9)-1-1.jpg]]<br> |
| - | [[File: | + | [[File:Igku Aug23 Gel Extraction(N9)-1-2.jpg]]<br> |
| + | |||
</div> | </div> | ||
Latest revision as of 16:08, 25 September 2013
Contents |
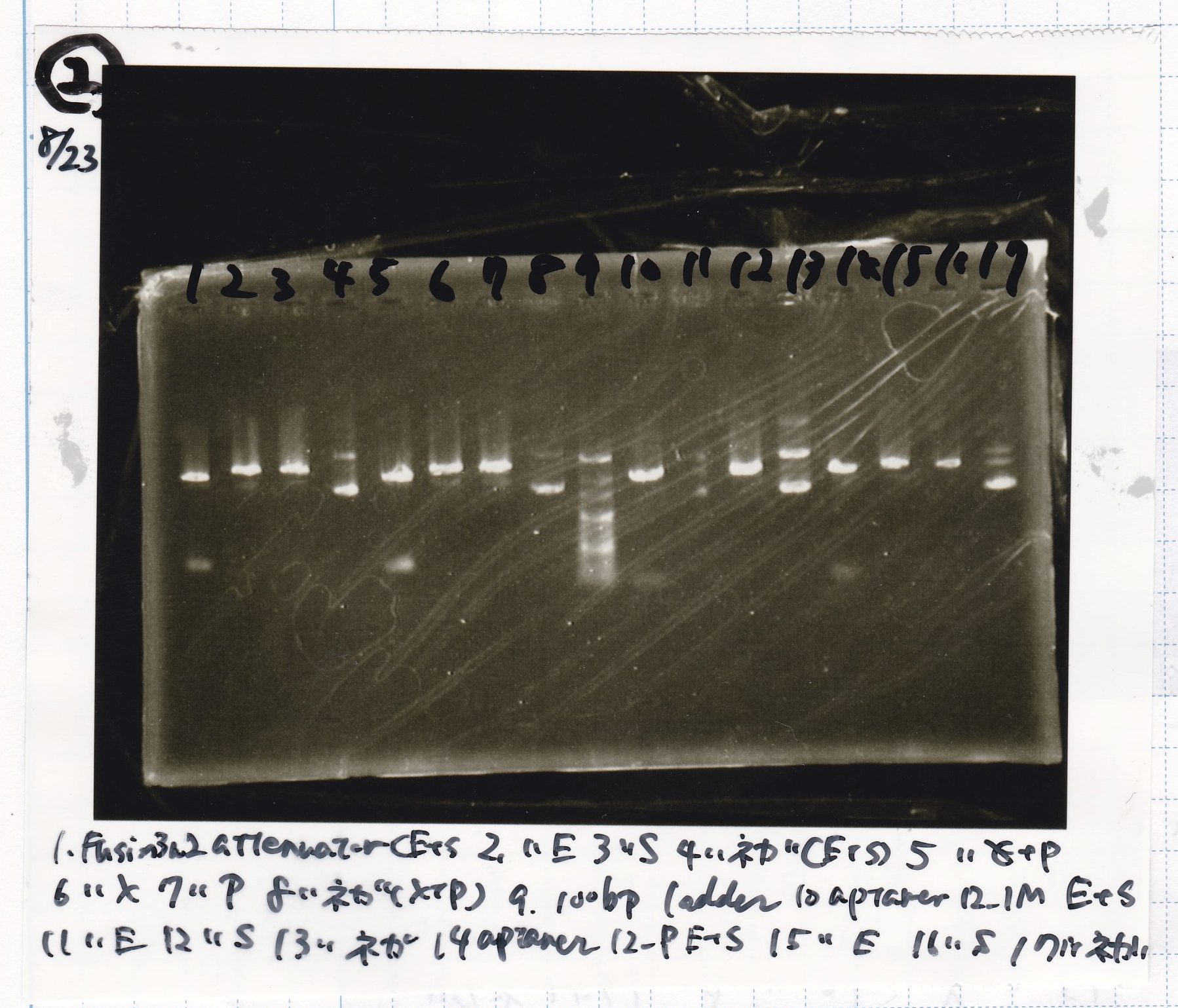
Aug 23
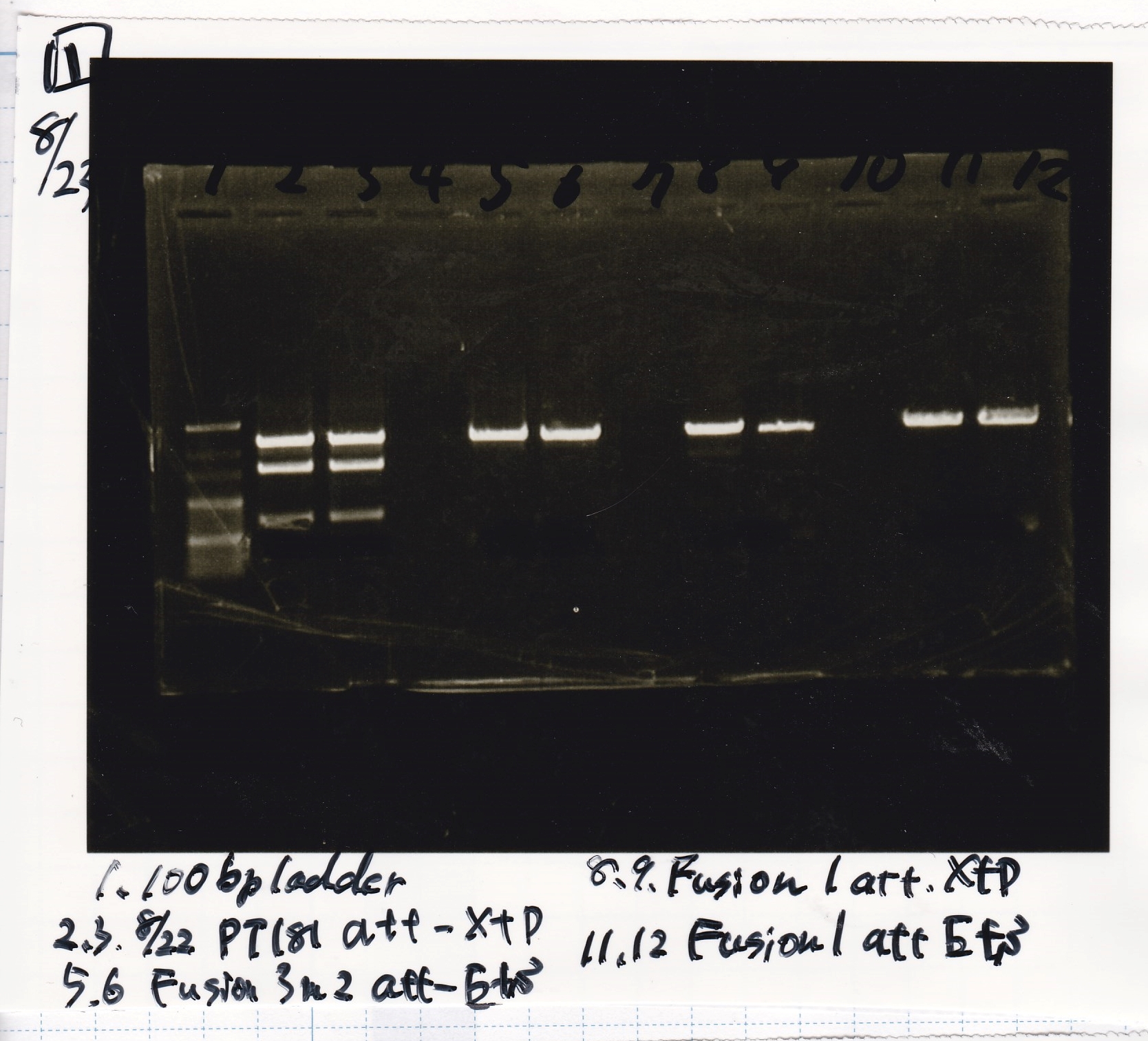
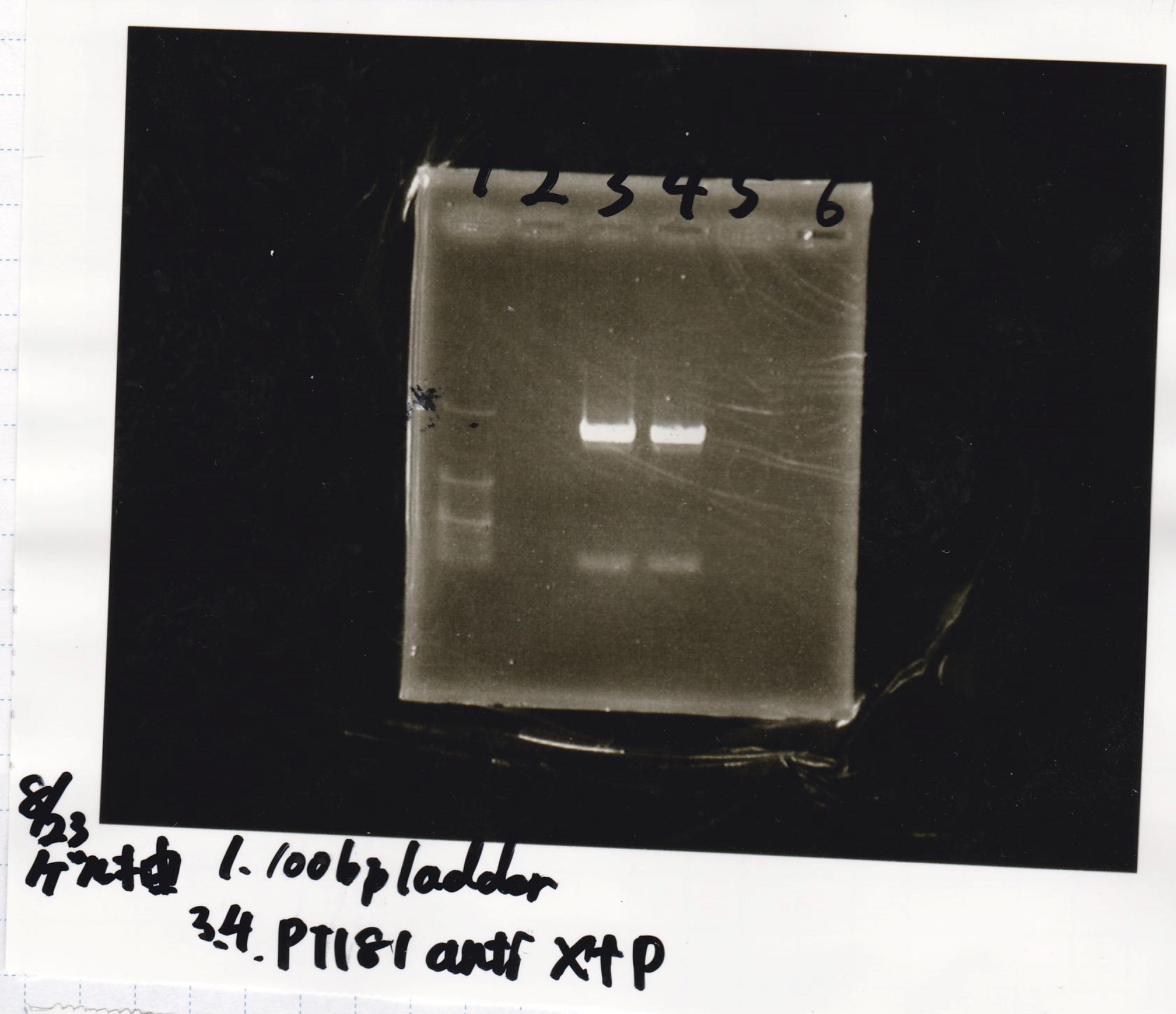
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | pT181 antisense | EcoRI | SpeI |
| 2 | pT181 antisense | EcoRI | -- |
| 3 | pT181 antisense | -- | SpeI |
| 4 | pT181 antisense | -- | -- |
| 5 | pT181 antisense | XbaI | PstI |
| 6 | pT181 antisense | XbaI | -- |
| 7 | pT181 antisense | -- | PstI |
| 8 | pT181 antisense | -- | -- |
| 9 | 100bp ladder | -- | -- |
| 10 | Fusion1 attenuator | EcoRI | SpeI |
| 11 | Fusion1 attenuator | EcoRI | -- |
| 12 | Fusion1 attenuator | -- | SpeI |
| 13 | Fusion1 attenuator | -- | -- |
| 14 | Fusion1 attenuator | XbaI | PstI |
| 15 | Fusion1 attenuator | XbaI | -- |
| 16 | Fusion1 attenuator | -- | PstI |
| 17 | Fusion1 attenuator | -- | -- |
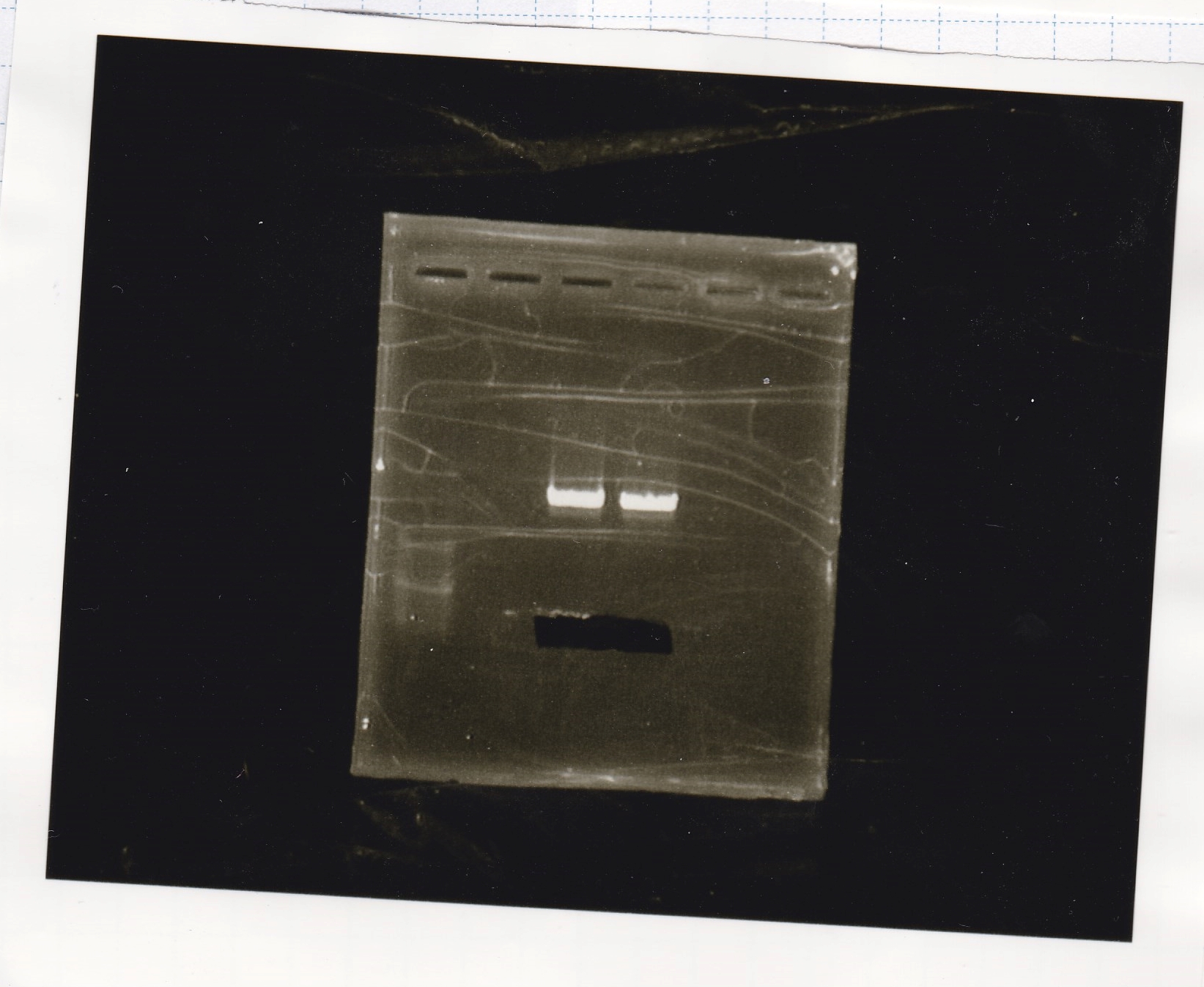
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | Fusion3m2 attenuator | EcoRI | SpeI |
| 2 | Fusion3m2 attenuator | EcoRI | -- |
| 3 | Fusion3m2 attenuator | -- | SpeI |
| 4 | Fusion3m2 attenuator | -- | -- |
| 5 | Fusion3m2 attenuator | XbaI | PstI |
| 6 | Fusion3m2 attenuator | XbaI | -- |
| 7 | Fusion3m2 attenuator | -- | PstI |
| 8 | Fusion3m2 attenuator | -- | -- |
| 9 | 100bp ladder | -- | -- |
| 10 | tetR aptamer 12_1M | EcoRI | SpeI |
| 11 | tetR aptamer 12_1M | EcoRI | -- |
| 12 | tetR aptamer 12_1M | -- | SpeI |
| 13 | tetR aptamer 12_1M | -- | -- |
| 14 | tetR aptamer 12_P | EcoRI | SpeI |
| 15 | tetR aptamer 12_P | EcoRI | -- |
| 16 | tetR aptamer 12_P | -- | SpeI |
| 17 | tetR aptamer 12_P | -- | -- |
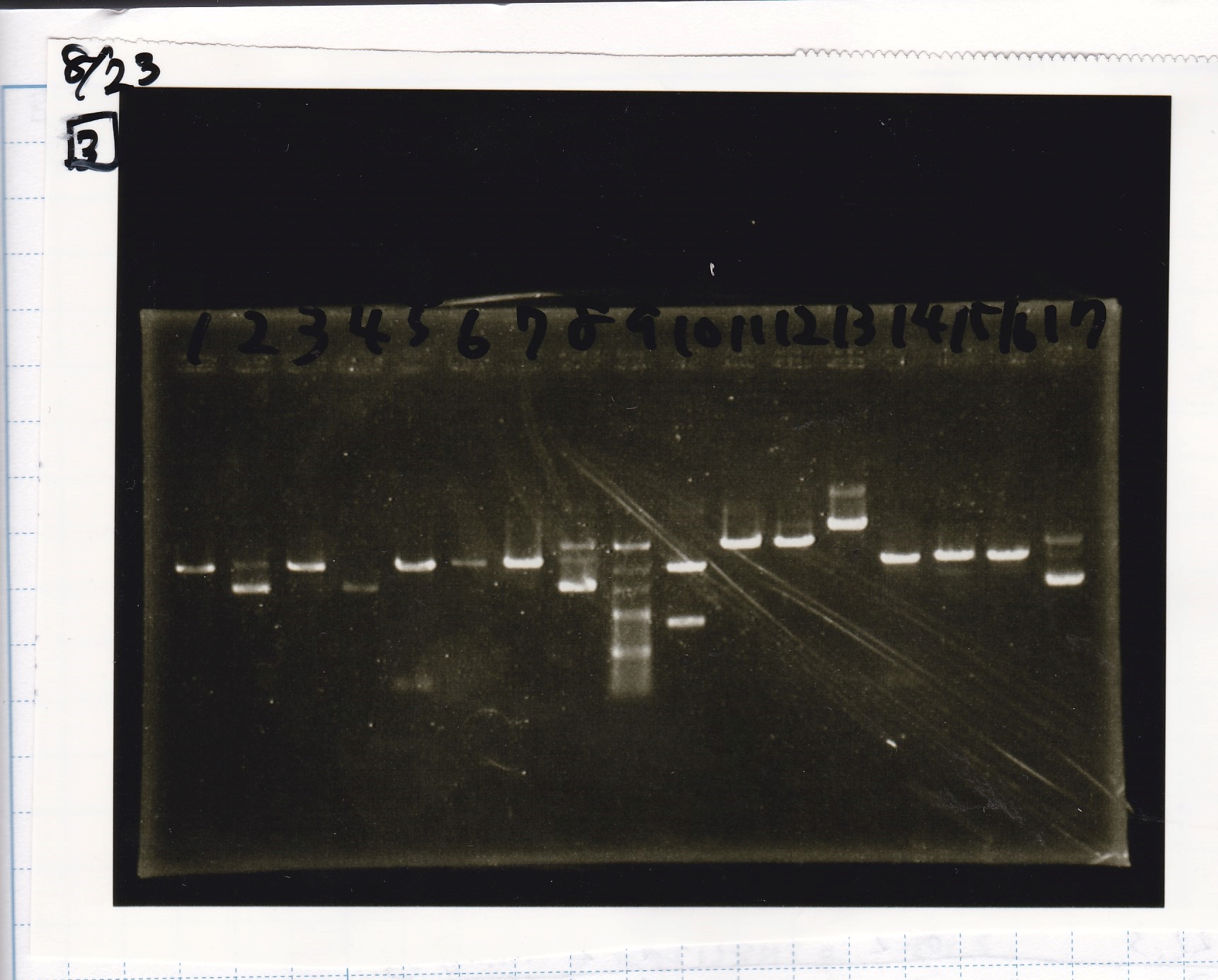
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | Plac | SpeI | PstI |
| 2 | Plac | SpeI | -- |
| 3 | Plac | -- | PstI |
| 4 | Plac | -- | -- |
| 5 | Spinach | EcoRI | SpeI |
| 6 | Spinach | EcoRI | -- |
| 7 | Spinach | -- | SpeI |
| 8 | Spinach | -- | -- |
| 9 | 100bp ladder | -- | -- |
| 10 | J23100 | SpeI | PstI |
| 11 | J23100 | SpeI | -- |
| 12 | J23100 | -- | PstI |
| 13 | J23100 | -- | -- |
| 14 | tetR aptamer 12_1R | EcoRI | SpeI |
| 15 | tetR aptamer 12_1R | EcoRI | -- |
| 16 | tetR aptamer 12_1R | -- | SpeI |
| 17 | tetR aptamer 12_1R | -- | -- |
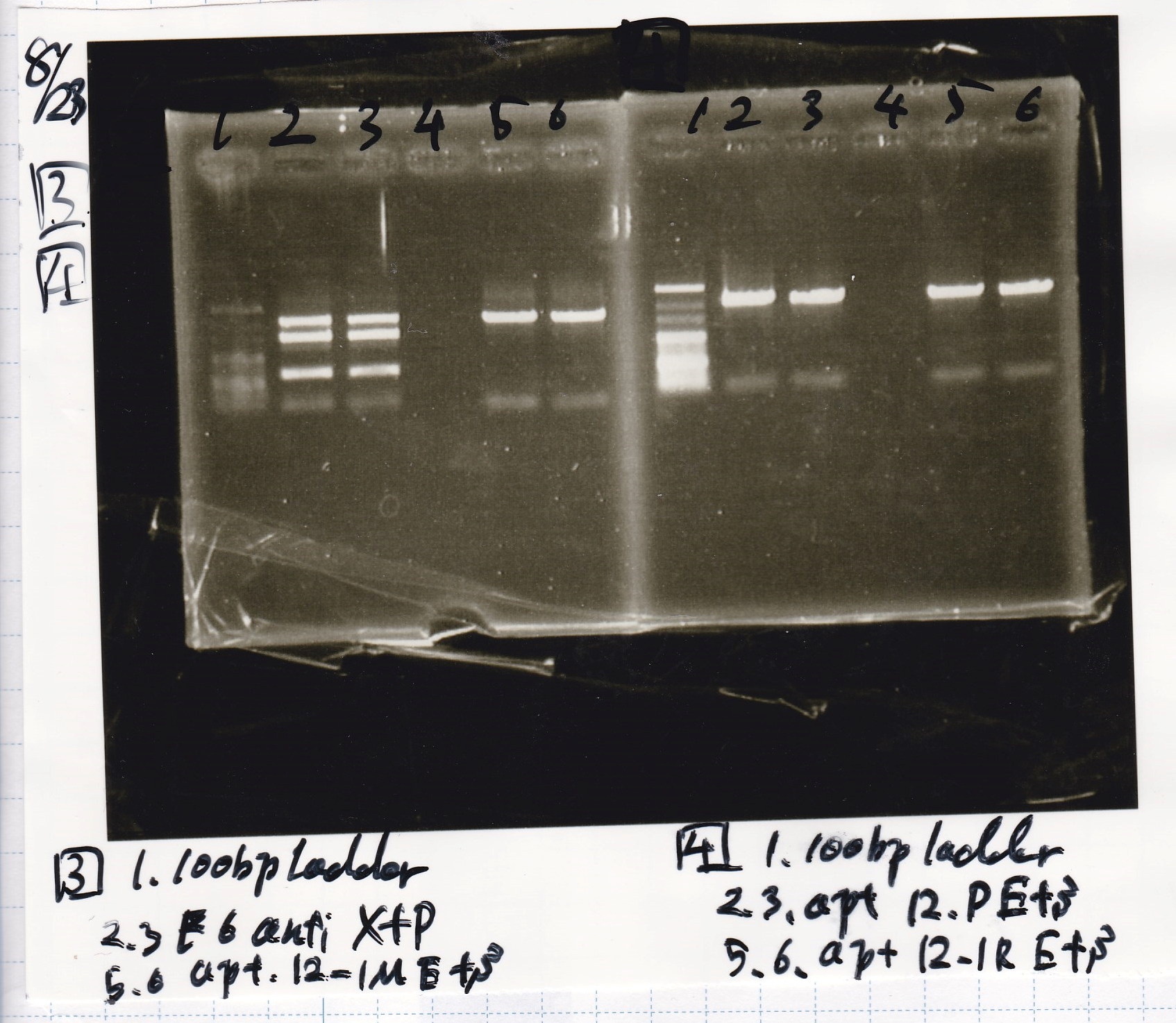
Restriction Enzyme Digestion
| Fusion6 antisense (1) | EcoRI | SpeI | buffer B | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 3 | 0.5 | 0.5 | 3 | 0.3 | 22.7 | 30 |
| 1 cut | 0.6 | 0.1 | 0 | 1 | 0.1 | 8.2 | 10 |
| 1 cut | 0.6 | 0 | 0.1 | 1 | 0.1 | 8.2 | 10 |
| NC | 0.6 | 0 | 0 | 1 | 0.1 | 8.3 | 10 |
| Fusion6 antisense (1) | XbaI | PstI | buffer D | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 3 | 0.5 | 0.5 | 3 | 0.3 | 22.7 | 30 |
| 1 cut | 0.6 | 0.1 | 0 | 1 | 0.1 | 8.2 | 10 |
| 1 cut | 0.6 | 0 | 0.1 | 1 | 0.1 | 8.2 | 10 |
| NC | 0.6 | 0 | 0 | 1 | 0.1 | 8.3 | 10 |
| Fusion1 antisense (1) | EcoRI | SpeI | buffer B | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 3.3 | 0.5 | 0.5 | 3 | 0.3 | 22.4 | 30 |
| 1 cut | 0.7 | 0.1 | 0 | 1 | 0.1 | 8.1 | 10 |
| 1 cut | 0.7 | 0 | 0.1 | 1 | 0.1 | 8.1 | 10 |
| NC | 0.7 | 0 | 0 | 1 | 0.1 | 8.2 | 10 |
| pT181 antisense (1) | EcoRI | SpeI | buffer B | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 5.6 | 0.5 | 0.5 | 3 | 0.3 | 20.1 | 30 |
| 1 cut | 1.1 | 0.1 | 0 | 1 | 0.1 | 7.7 | 10 |
| 1 cut | 1.1 | 0 | 0.1 | 1 | 0.1 | 7.7 | 10 |
| NC | 1.1 | 0 | 0 | 1 | 0.1 | 7.8 | 10 |
| pT181 antisense (1) | XbaI | PstI | buffer D | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 5.6 | 0.5 | 0.5 | 3 | 0.3 | 20.1 | 30 |
| 1 cut | 1.1 | 0.1 | 0 | 1 | 0.1 | 7.7 | 10 |
| 1 cut | 1.1 | 0 | 0.1 | 1 | 0.1 | 7.7 | 10 |
| NC | 1.1 | 0 | 0 | 1 | 0.1 | 7.8 | 10 |
- incubate at 37°C from 17:29.
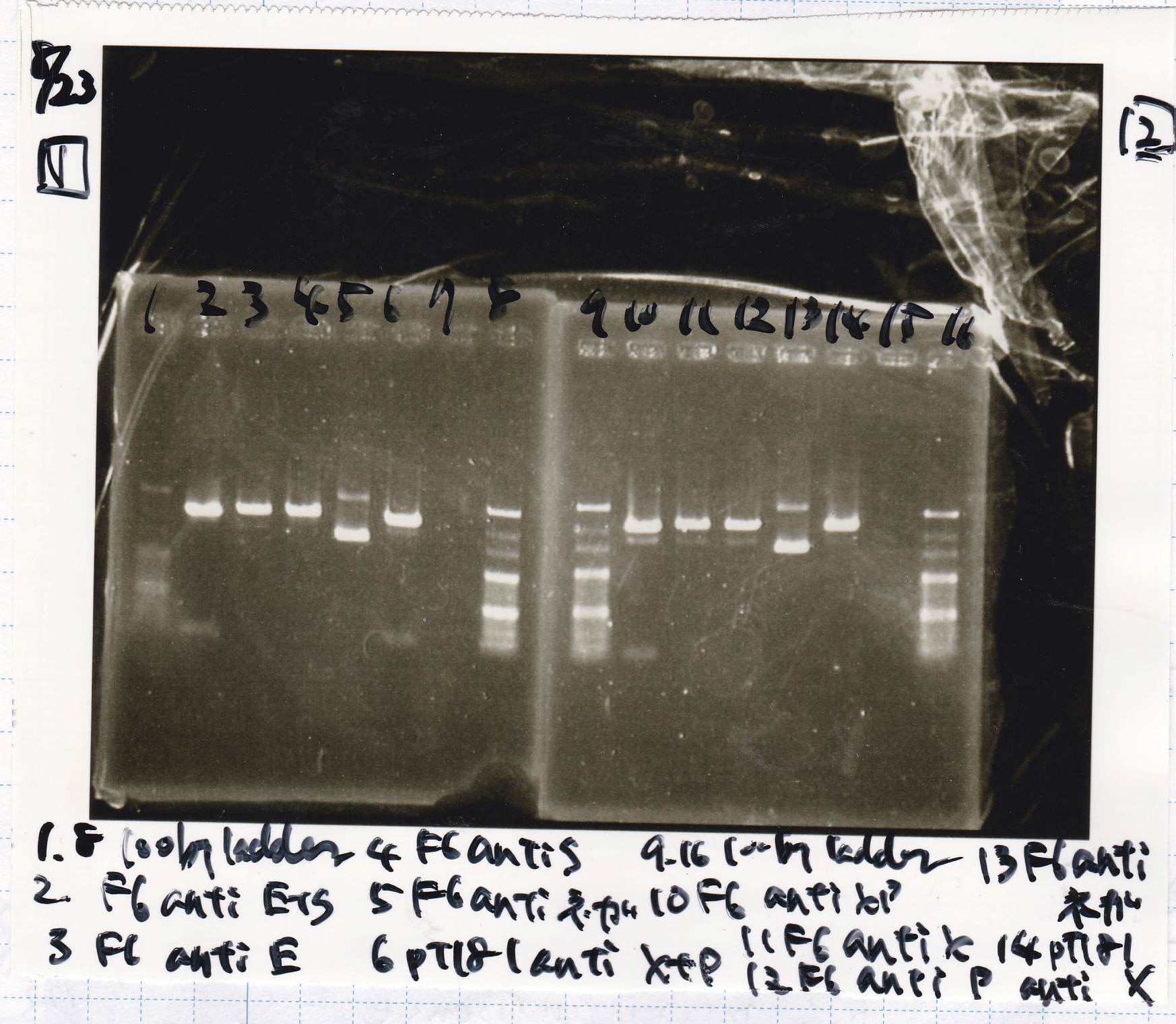
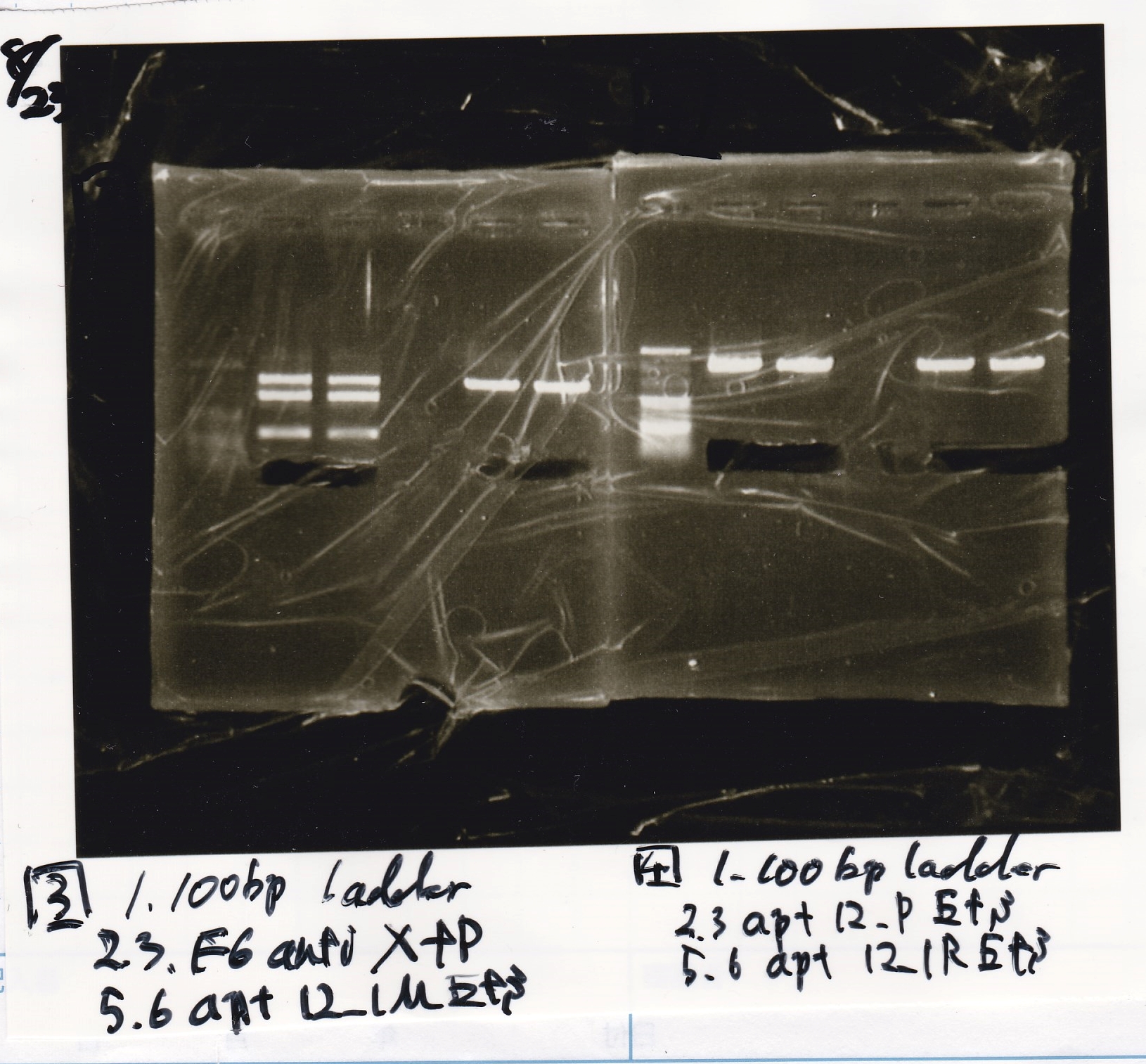
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp | -- | -- |
| 2 | pT181 attenuator | EcoRI | SpeI |
| 3 | pT181 attenuator | XbaI | PstI |
| 4 | Fusion3m2 attenuator | EcoRI | SpeI |
| 5 | Fusion1 attenuator | XbaI | PstI |
| 6 | Fusion1 attenuator | EcoRI | SpeI |
| 7 | Fusion3m2 attenuator | XbaI | PstI |
| 8 | pT181 antisense | EcoRI | SpeI |
| 9 | pT181 antisense | XbaI | PstI |
| 10 | Fusion1 antisense | EcoRI | SpeI |
| 11 | Fusion1 antisense | XbaI | PstI |
| 12 | Fusion6 antisense | EcoRI | SpeI |
| 13 | Fusion6 antisense | XbaI | PstI |
| 14 | tetR aptamer 12_1M | EcoRI | SpeI |
| 15 | tetR aptamer 12_P | EcoRI | SpeI |
| 16 | tetR aptamer 12_1R | EcoRI | SpeI |
| 17 | 100bp | -- | -- |
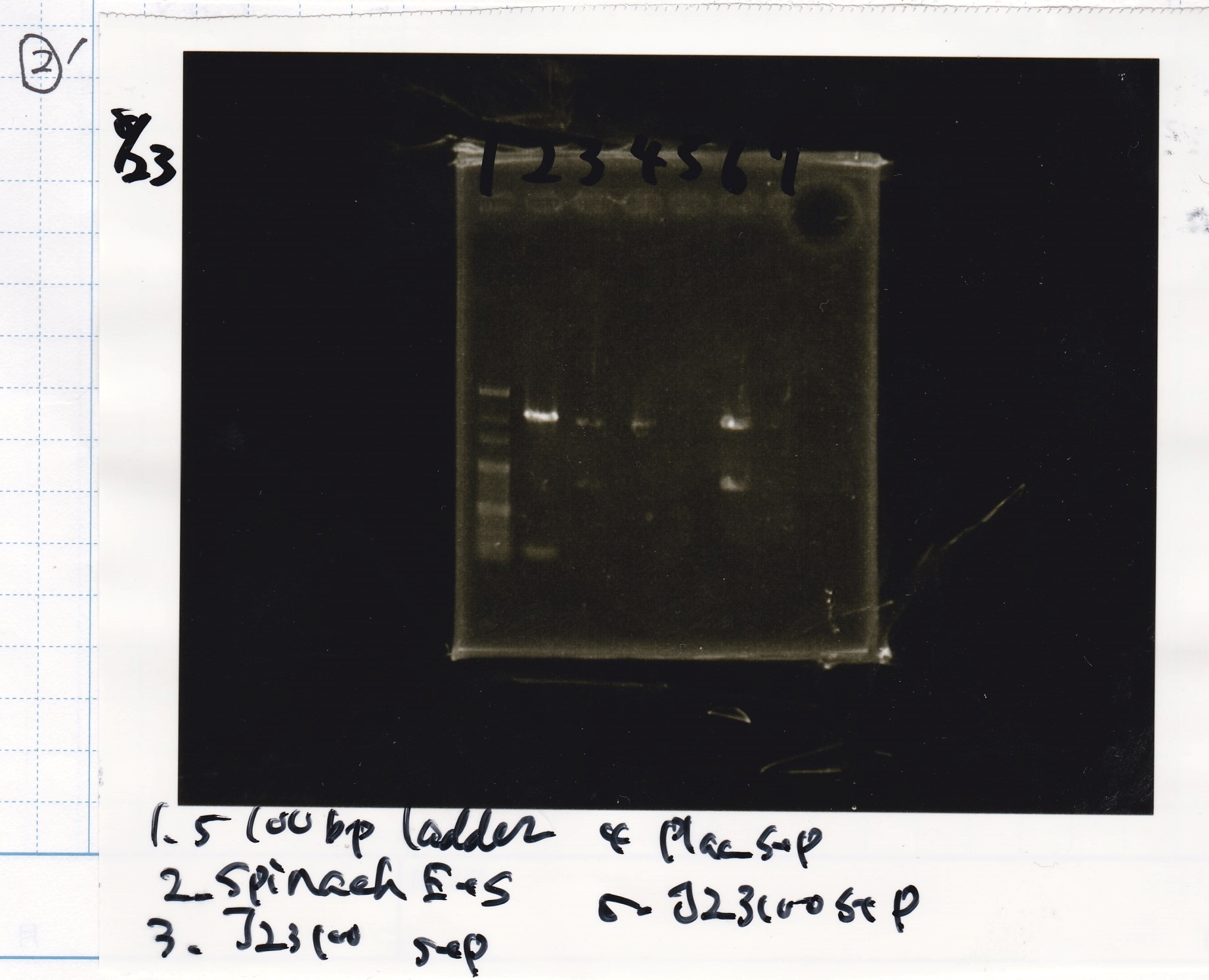
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp | -- | -- |
| 2 | Spinach | EcoRI | SpeI |
| 3 | J23100 | SpeI | PstI |
| 4 | Plac | SpeI | PstI |
| 5 | 100bp | -- | -- |
| 6 | J23100 | SpeI | PstI |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp | -- | -- |
| 2 | Fusion6 antisense | EcoRI | SpeI |
| 3 | Fusion6 antisense | EcoRI | -- |
| 4 | Fusion6 antisense | -- | SpeI |
| 5 | Fusion6 antisense | -- | -- |
| 6 | pT181 antisense | XbaI | PstI |
| 8 | 100bp | -- | -- |
| 9 | 100bp | -- | -- |
| 10 | Fusion6 antisense | XbaI | PstI |
| 11 | Fusion6 antisense | XbaI | -- |
| 12 | Fusion6 antisense | -- | PstI |
| 13 | Fusion6 antisense | -- | -- |
| 14 | pT181 antisense | XbaI | -- |
| 16 | 100bp | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp | -- | -- |
| 2 | Fusion1 antisense | EcoRI | SpeI |
| 3 | Fusion1 antisense | EcoRI | -- |
| 4 | Fusion1 antisense | -- | SpeI |
| 5 | Fusion1 antisense | -- | -- |
| 6 | pT181 antisense | -- | PstI |
| 8 | 100bp | -- | -- |
| 9 | 100bp | -- | -- |
| 10 | pT181 antisense | EcoRI | SpeI |
| 11 | pT181 antisense | EcoRI | -- |
| 12 | pT181 antisense | -- | SpeI |
| 13 | pT181 antisense | -- | -- |
| 14 | pT181 antisense | -- | -- |
| 16 | 100bp | -- | -- |
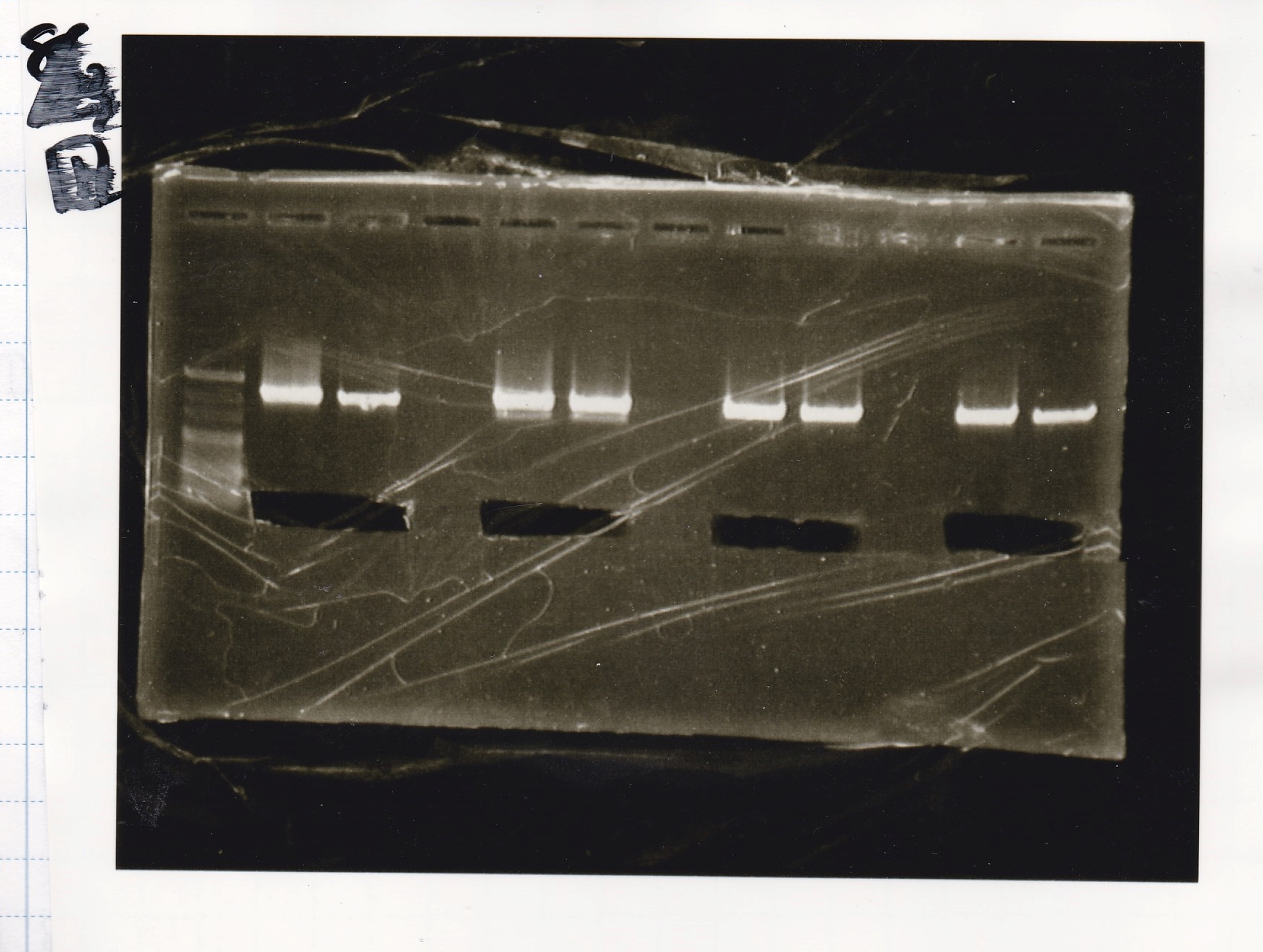
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 2 | pT181 attenuator | XbaI & PstI |
| 3 | ||
| 5 | Fusion3m2 attenuator | EcoRI & SpeI |
| 6 | ||
| 8 | Fusion1 attenuator | XbaI & PstI |
| 9 | ||
| 11 | Fusion1 attenuator | EcoRI & SpeI |
| 12 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 2 | Fusion3m2 attenuator | XbaI & PstI |
| 3 | ||
| 5 | pT181 antisense | EcoRI & SpeI |
| 6 | ||
| 8 | pT181 antisense | XbaI & PstI |
| 9 | ||
| 11 | Fusion1 antisense | XbaI & PstI |
| 12 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 2 | Fusion6 attenuator | XbaI & PstI |
| 3 | ||
| 5 | aptamer 12_1M | EcoRI & SpeI |
| 6 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 2 | aptamer 12_P | EcoRI & SpeI |
| 3 | ||
| 5 | aptamer 12_1R | EcoRI & SpeI |
| 6 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 2 | Fusion6 antisense | EcoRI & SpeI |
| 3 | ||
| 5 | Fusion6 attenuator | XbaI & PstI |
| 6 | ||
| 8 | Fusion1 antisense | EcoRI & SpeI |
| 9 | ||
| 11 | pT181 antisense | EcoRI & SpeI |
| 12 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 3 | pT181 antisense | XbaI & PstI |
| 4 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 3 | Plac | SpeI & PstI |
| 4 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 3 | J23100 | SpeI & PstI |
| 5 | Spinach | EcoRI & SpeI |
| 6 |
 "
"