Template:Kyoto/Notebook/Aug 28
From 2013.igem.org
(Difference between revisions)
(→Colony PCR) |
(→Liquid Culture) |
||
| (19 intermediate revisions not shown) | |||
| Line 20: | Line 20: | ||
|7||100bp | |7||100bp | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug28 Electrophoresis(N①-1).jpg]]<br> |
</div> | </div> | ||
| Line 39: | Line 39: | ||
!state||colspan="2"|Vector||colspan="2"|Inserter | !state||colspan="2"|Vector||colspan="2"|Inserter | ||
|- | |- | ||
| - | |experiment||8/ | + | |experiment||8/17 DT-1||3.0||8/20 RBS-lysis3-1|8.0 |
|} | |} | ||
*Samples were evaporeted used evaporator into about 3 µL. | *Samples were evaporeted used evaporator into about 3 µL. | ||
| Line 67: | Line 67: | ||
|9||PkaiBC(8/27 Genome PCR production) | |9||PkaiBC(8/27 Genome PCR production) | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug28 Gel Extraction(N②pic)before.jpg]]<br> |
| - | [[File: | + | [[File:Igku Aug28 Gel Extraction(N①pic)after.jpg]]<br> |
| + | |||
| + | {| class="wikitable" | ||
| + | !Name||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |RpaA||15.4||1.88||0.03 | ||
| + | |- | ||
| + | |SasA||21.6||1.66||0.79 | ||
| + | |- | ||
| + | |PkaiBC||16.3||1.79||0.68 | ||
| + | |- | ||
| + | |RpaB||26.7||1.77||0.82 | ||
| + | |} | ||
===Colony PCR=== | ===Colony PCR=== | ||
| Line 91: | Line 103: | ||
|5min||30s||30s||48s||30cycles | |5min||30s||30s||48s||30cycles | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug28 Colony PCR(N③pic).jpg]] |
</div> | </div> | ||
| Line 159: | Line 171: | ||
|4||8/17 RBS-GFP-DT-(2)||--||-- | |4||8/17 RBS-GFP-DT-(2)||--||-- | ||
|- | |- | ||
| - | |5||8/20 Pcon-RBS-GFP-DT-(1)||EcoRI|| | + | |5||8/20 Pcon-RBS-GFP-DT-(1)||EcoRI||SpeI |
|- | |- | ||
|6||8/20 Pcon-RBS-GFP-DT-(1)||--||-- | |6||8/20 Pcon-RBS-GFP-DT-(1)||--||-- | ||
| Line 177: | Line 189: | ||
|13||8/20 RBS-tetR-DT||--||-- | |13||8/20 RBS-tetR-DT||--||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug28 Electrophoresis(ColoP)(N4)pic.jpg]]<br> |
</div> | </div> | ||
| Line 198: | Line 210: | ||
|6 | |6 | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug28 Gel Extraction(N⑤-1,2)before.jpg]]<br> |
| - | [[File: | + | [[File:Igku Aug28 Gel Extraction4-2.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Lane||DNA||Enzyme | !Lane||DNA||Enzyme | ||
| Line 215: | Line 227: | ||
|6 | |6 | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug28 Gel Extraction(N⑤-3).jpg]]<br> |
| - | + | ||
{| class="wikitable" | {| class="wikitable" | ||
!Name||concentration[µg/mL]||260/280||260/230 | !Name||concentration[µg/mL]||260/280||260/230 | ||
| Line 257: | Line 268: | ||
|5min||30s||30s||30s||30cycles | |5min||30s||30s||30s||30cycles | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug28 c(N⑤-1,2)before.jpg]] |
{| class="wikitable" | {| class="wikitable" | ||
!|Sample||base pair | !|Sample||base pair | ||
|- | |- | ||
| - | |8/27 | + | |8/27 Pλ-luxI-1||-- |
|- | |- | ||
| - | |8/27 | + | |8/27 Pλ-luxI||-- |
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 272: | Line 283: | ||
|5min||30s||30s||30min||3cycles | |5min||30s||30s||30min||3cycles | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug28 ColonyPCR(N⑦-2).jpg]] |
</div> | </div> | ||
| + | |||
| + | ===Colony PCR=== | ||
| + | <div class="expeiment"> | ||
| + | <span class>No name</span> | ||
| + | {| class="wikitable" | ||
| + | !|Sample||base pair | ||
| + | |- | ||
| + | |8/21 RBS-lysis2-9||772 | ||
| + | |- | ||
| + | |8/21 RBS-lysis2-10||772 | ||
| + | |- | ||
| + | |8/21 RBS-lysis2-11||772 | ||
| + | |- | ||
| + | |8/21 RBS-lysis2-12||772 | ||
| + | |- | ||
| + | |8/21 RBS-lysis2-13||772 | ||
| + | |- | ||
| + | |8/21 RBS-lysis2-14||772 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94°C||94°C||55°C||68°C||-- | ||
| + | |- | ||
| + | |5min||30s||30s||48s||30cycles | ||
| + | |} | ||
===Liquid Culture=== | ===Liquid Culture=== | ||
| Line 283: | Line 320: | ||
|8/16 Master Plate(CP)-6 DT||Plusgrow medium(+CP) | |8/16 Master Plate(CP)-6 DT||Plusgrow medium(+CP) | ||
|- | |- | ||
| - | | | + | |J23100 Master Plate-1||Plusgrow medium(+Amp) |
|- | |- | ||
| - | |Plac Master Plate-1||Plusgrow medium(+CP) | + | |Plac Master Plate-1||Plusgrow medium(+CP) |
|} | |} | ||
</div> | </div> | ||
| Line 297: | Line 334: | ||
|experiment||8/27 DT (EcoRI & XbaI)||2.9||8/28 RBS-lysis1 (EcoRI & SpeI)||9.5 | |experiment||8/27 DT (EcoRI & XbaI)||2.9||8/28 RBS-lysis1 (EcoRI & SpeI)||9.5 | ||
|- | |- | ||
| - | |experiment||8/28 Plux (SpeI & PstI)||2.3||8/28 RBS-GFP-DT (XbaI & PstI)|| | + | |experiment||8/28 Plux (SpeI & PstI)||2.3||8/28 RBS-GFP-DT (XbaI & PstI)||18 |
|- | |- | ||
| - | |experiment||8/28 Pcon-RBS-luxR-DT (EcoRI&XbaI)||3.3||8/28 Pcon^RBS-GFP-DT-(1) (EcoRI&SpeI)||28 | + | |experiment||8/28 Pcon-RBS-luxR-DT (EcoRI & XbaI)||3.3||8/28 Pcon^RBS-GFP-DT-(1) (EcoRI & SpeI)||28 |
|- | |- | ||
| - | |8/27 DT (EcoRI&XbaI)||2.7||8/24 Spinach (EcoRI&SpeI)||2.4 | + | |experiment||8/27 DT (EcoRI & XbaI)||2.7||8/24 Spinach (EcoRI & SpeI)||2.4 |
|} | |} | ||
*Samples were evaporeted used evaporator into about 1 µL. | *Samples were evaporeted used evaporator into about 1 µL. | ||
| Line 318: | Line 355: | ||
!Sample||medium | !Sample||medium | ||
|- | |- | ||
| - | |8/26 | + | |8/26 Pλ-RBS-luxI-DT-1||Plusgrow medium (+Amp) |
|- | |- | ||
| - | |8/26 | + | |8/26 Pλ-RBS-luxI-DT-2||Plusgrow medium (+Amp) |
|} | |} | ||
* incubate 37°C 10hour | * incubate 37°C 10hour | ||
</div> | </div> | ||
Latest revision as of 03:46, 28 September 2013
Contents |
Aug 28
Electrophoresis
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 8/27 Plux | 173.8 | 1.95 | 1.45 |
Ligation
| state | Vector | Inserter | ||
|---|---|---|---|---|
| experiment | 8/17 DT-1 | 3.0 | 8.0 | |
- Samples were evaporeted used evaporator into about 3 µL.
| sample | MilliQ | Ligation High | total |
|---|---|---|---|
| 3 | 4 | 3.5 | 10.5 |
- incubate one hour at 16 °C
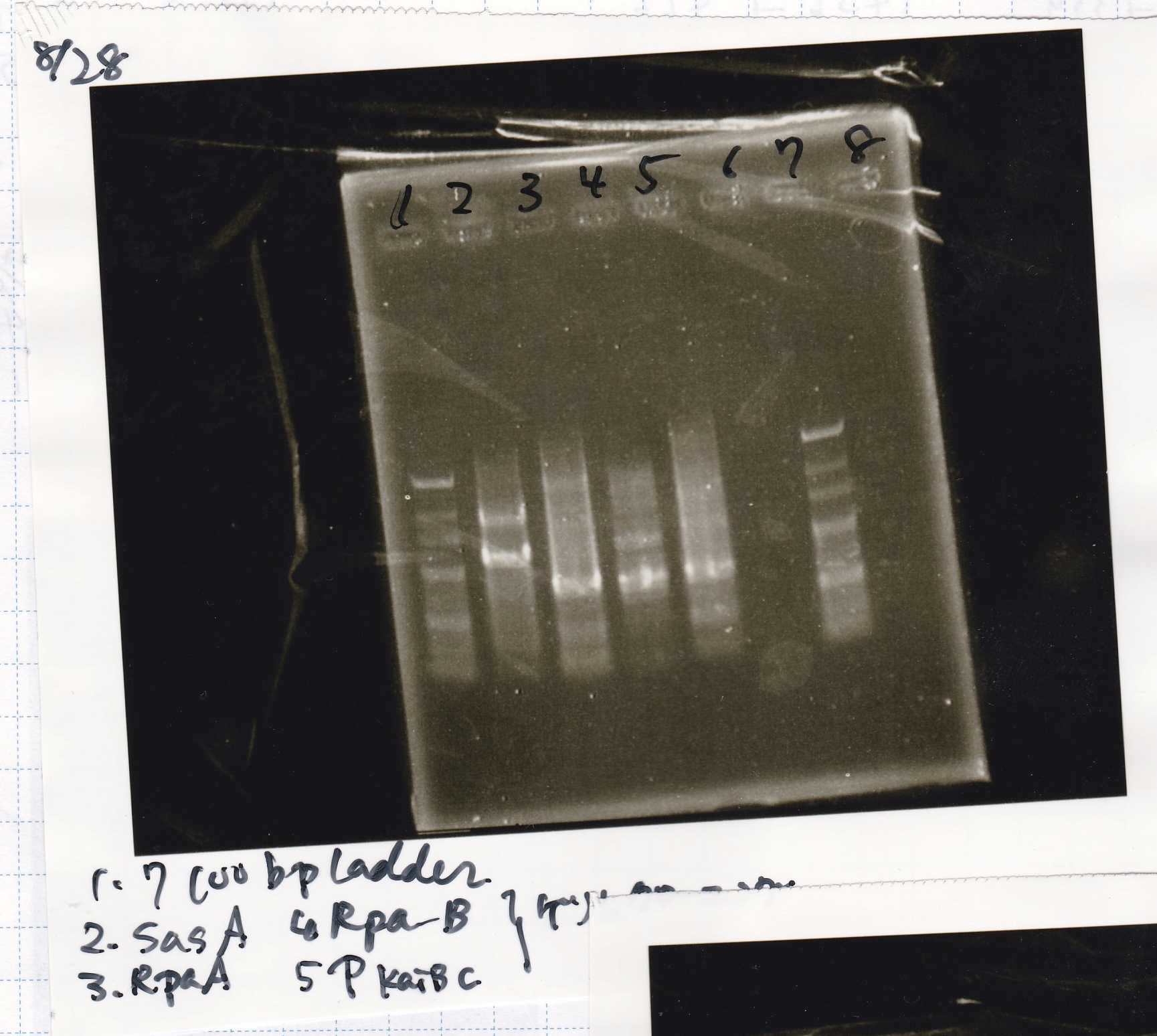
Gel Extraction
| Lane | DNA |
|---|---|
| 1 | 100bpladder |
| 3 | SasA(8/27 Genome PCR production) |
| 5 | RpaA(8/27 Genome PCR production) |
| 7 | RpaB(8/27 Genome PCR production) |
| 9 | PkaiBC(8/27 Genome PCR production) |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| RpaA | 15.4 | 1.88 | 0.03 |
| SasA | 21.6 | 1.66 | 0.79 |
| PkaiBC | 16.3 | 1.79 | 0.68 |
| RpaB | 26.7 | 1.77 | 0.82 |
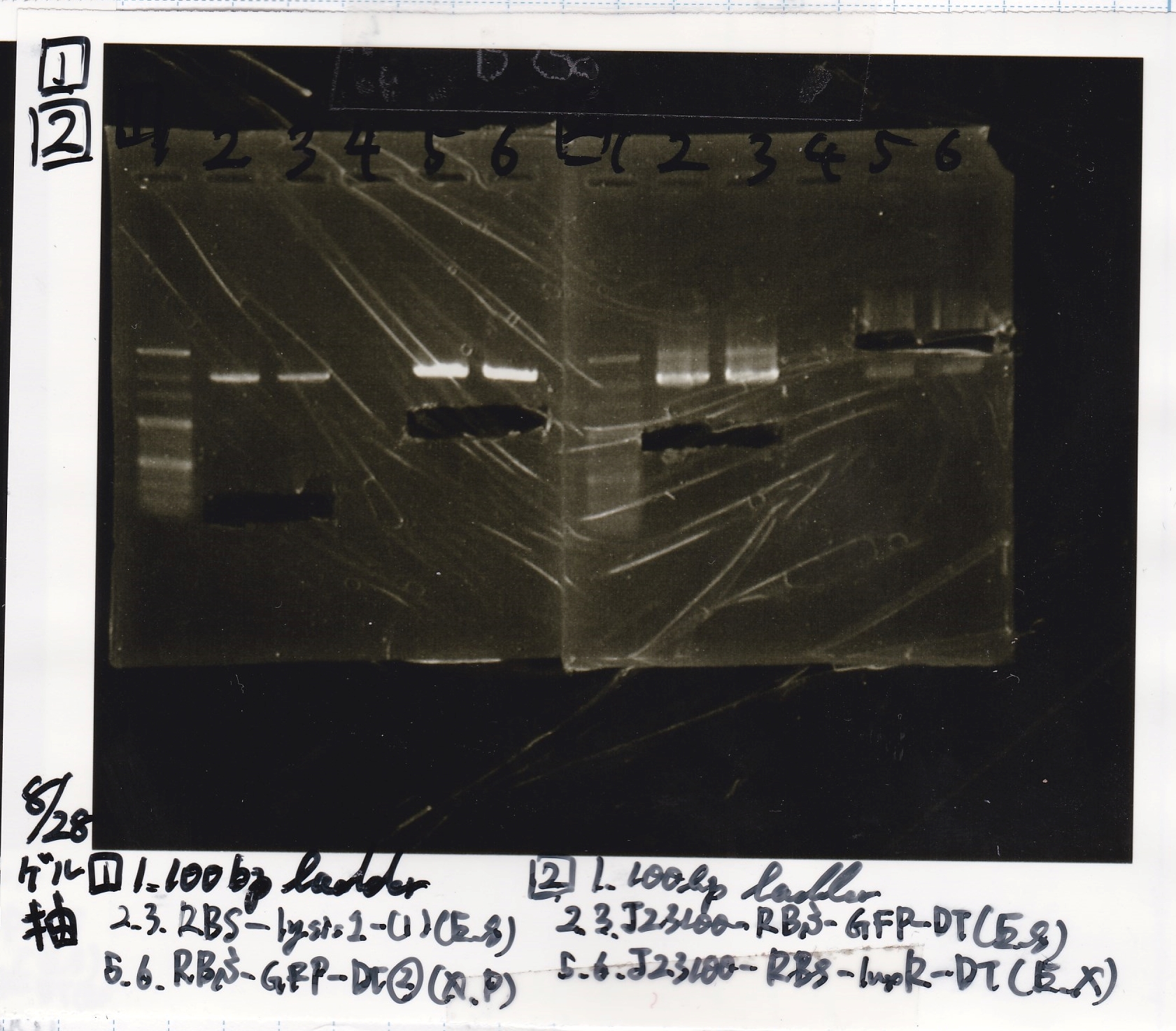
Colony PCR
| Sample | base pair |
|---|---|
| 8/21 RBS-lysis2-(3) | 772 |
| 8/21 RBS-lysis2-(4) | 772 |
| 8/21 RBS-lysis2-(5) | 772 |
| 8/21 RBS-lysis2-(6) | 772 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 48s | 30cycles |
Restriction Enzyme Digestion
| 8/22 RBS-lysis1-(1) | EcoRI | SpeI | BufferB | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 9µL | 0.5µL | 0.5µL | 3µL | 0.3µL | 16.7µL | 30µL |
| NC | 1µL | 0µL | 0µL | 1µL | 0.1µL | 7.9µL | 10µL |
| 8/17 RBS-GFP-DT-(2) | XbaI | PstI | BufferD | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 4µL | 0.5µL | 0.5µL | 3µL | 0.3µL | 21.7µL | 30µL |
| NC | 0.5µL | 0µL | 0µL | 1µL | 0.1µL | 8.4µL | 10µL |
| 8/20 Pcon-RBS-GFP-DT-(1) | EcoRI | SpeI | BufferB | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 3µL | 0.5µL | 0.5µL | 3µL | 0.3µL | 22.7µL | 30µL |
| NC | 0.3µL | 0µL | 0µL | 1µL | 0.1µL | 8.6µL | 10µL |
| 8/20 Pcon-RBS-luxR-DT-(1) | EcoRI | XbaI | BufferD | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 2µL | 0.5µL | 0.5µL | 3µL | 0.3µL | 23.7µL | 30µL |
| NC | 0.3µL | 0µL | 0µL | 1µL | 0.1µL | 8.6µL | 10µL |
| 8/28 Plux | SpeI | PstI | BufferD | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 6µL | 0.5µL | 0.5µL | 3µL | 0.3µL | 19.7µL | 30µL |
| NC | 1µL | 0µL | 0µL | 1µL | 0.1µL | 7.9µL | 10µL |
| 8/20 RBS-tetR-DT-(1) | XbaI | PstI | BufferD | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 5µL | 0.5µL | 0.5µL | 3µL | 0.3µL | 20.7µL | 30µL |
| NC | 0.5µL | 0µL | 0µL | 1µL | 0.1µL | 8.4µL | 10µL |
- incubated 37°C 1hour
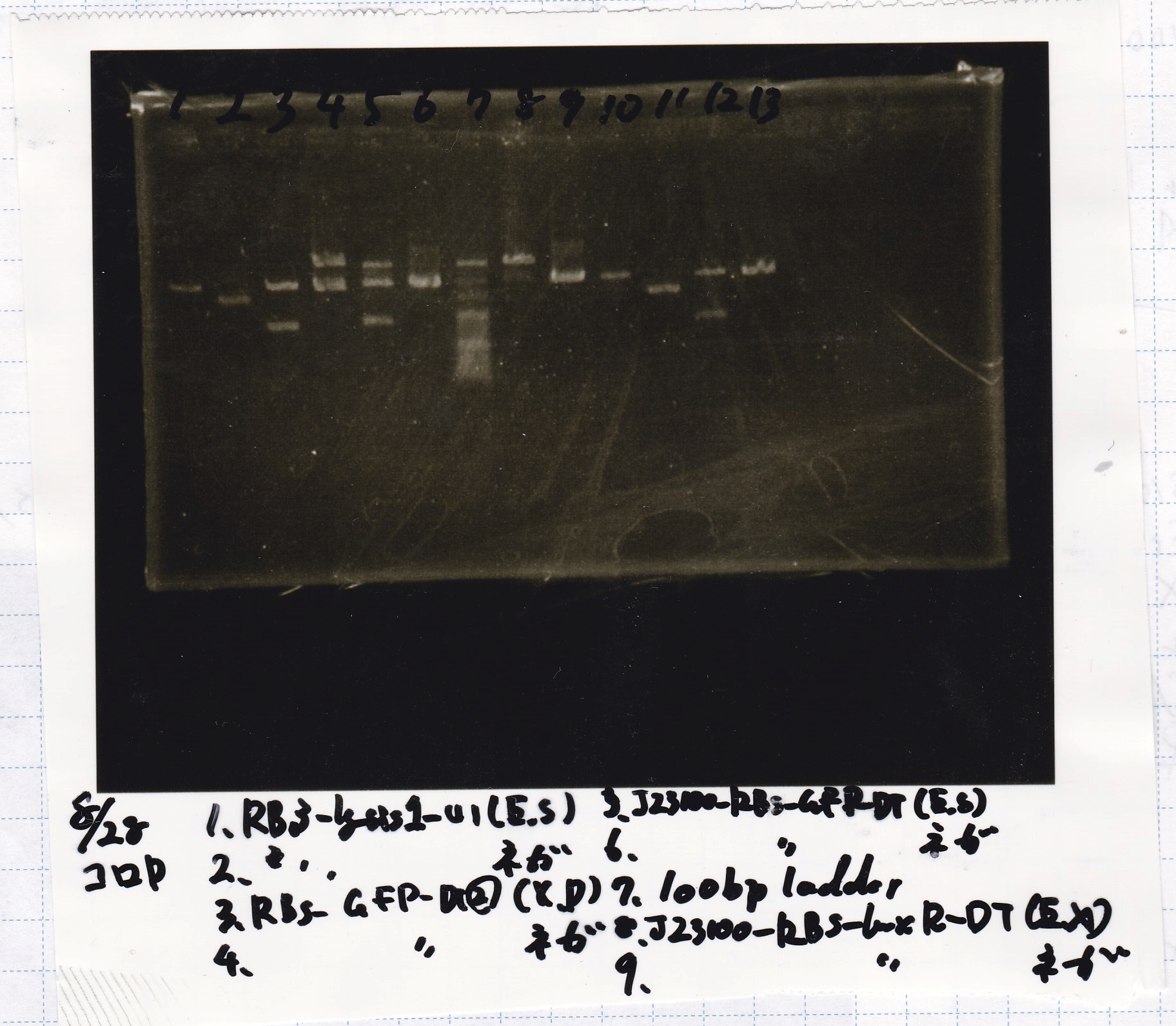
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 8/22 RBS-lysis1-(1) | EcoRI | SpeI |
| 2 | 8/22 RBS-lysis1-(1) | -- | -- |
| 3 | 8/17 RBS-GFP-DT-(2) | XbaI | PstI |
| 4 | 8/17 RBS-GFP-DT-(2) | -- | -- |
| 5 | 8/20 Pcon-RBS-GFP-DT-(1) | EcoRI | SpeI |
| 6 | 8/20 Pcon-RBS-GFP-DT-(1) | -- | -- |
| 7 | 100bp ladder | -- | -- |
| 8 | 8/20 Pcon-RBS-luxR-DT-(1) | EcoRI | XbaI |
| 9 | 8/20 Pcon-RBS-luxR-DT-(1) | -- | -- |
| 10 | 8/28 Plux | SpeI | PstI |
| 11 | 8/28 Plux | -- | -- |
| 12 | 8/20 RBS-tetR-DT | XbaI | PstI |
| 13 | 8/20 RBS-tetR-DT | -- | -- |
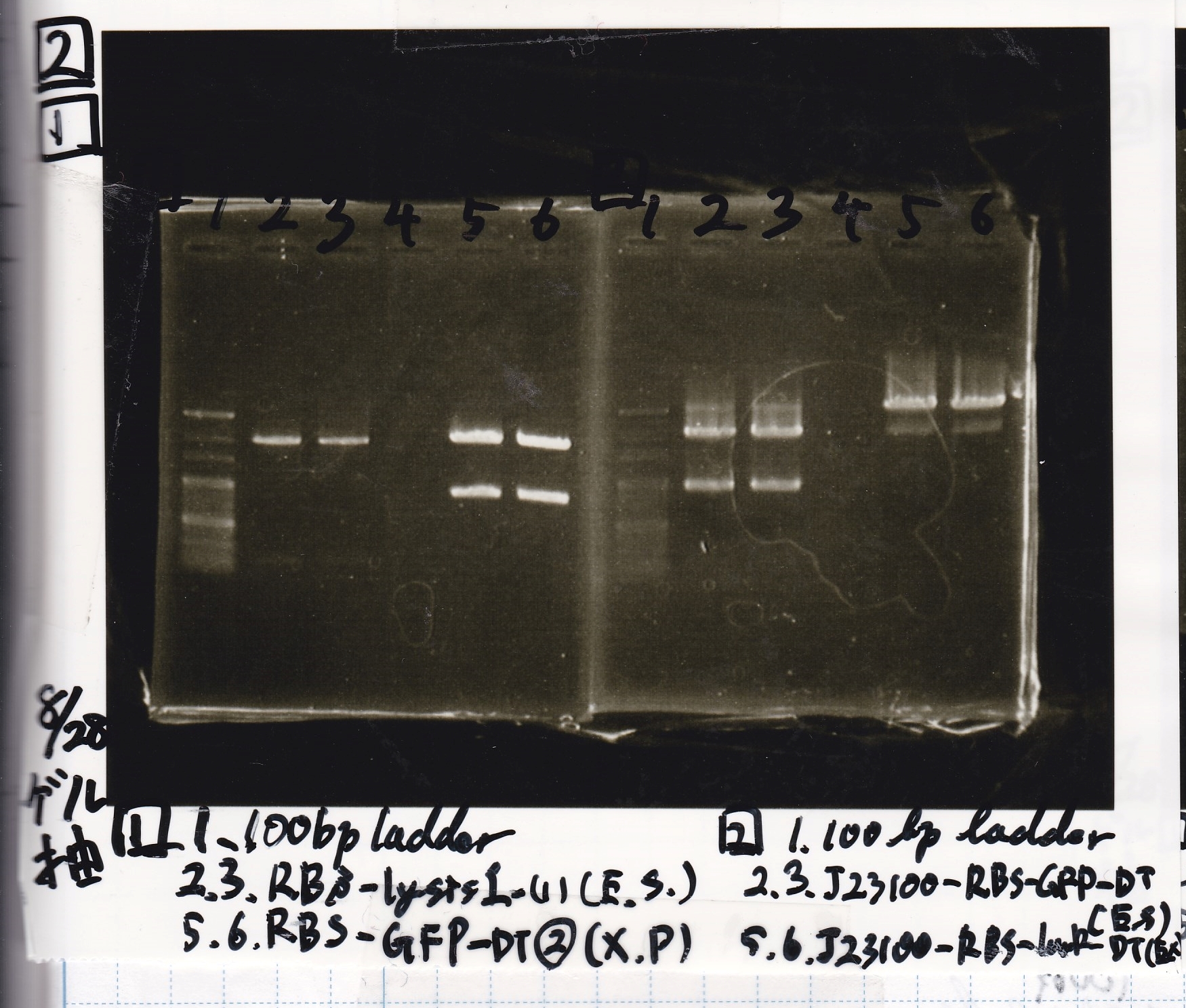
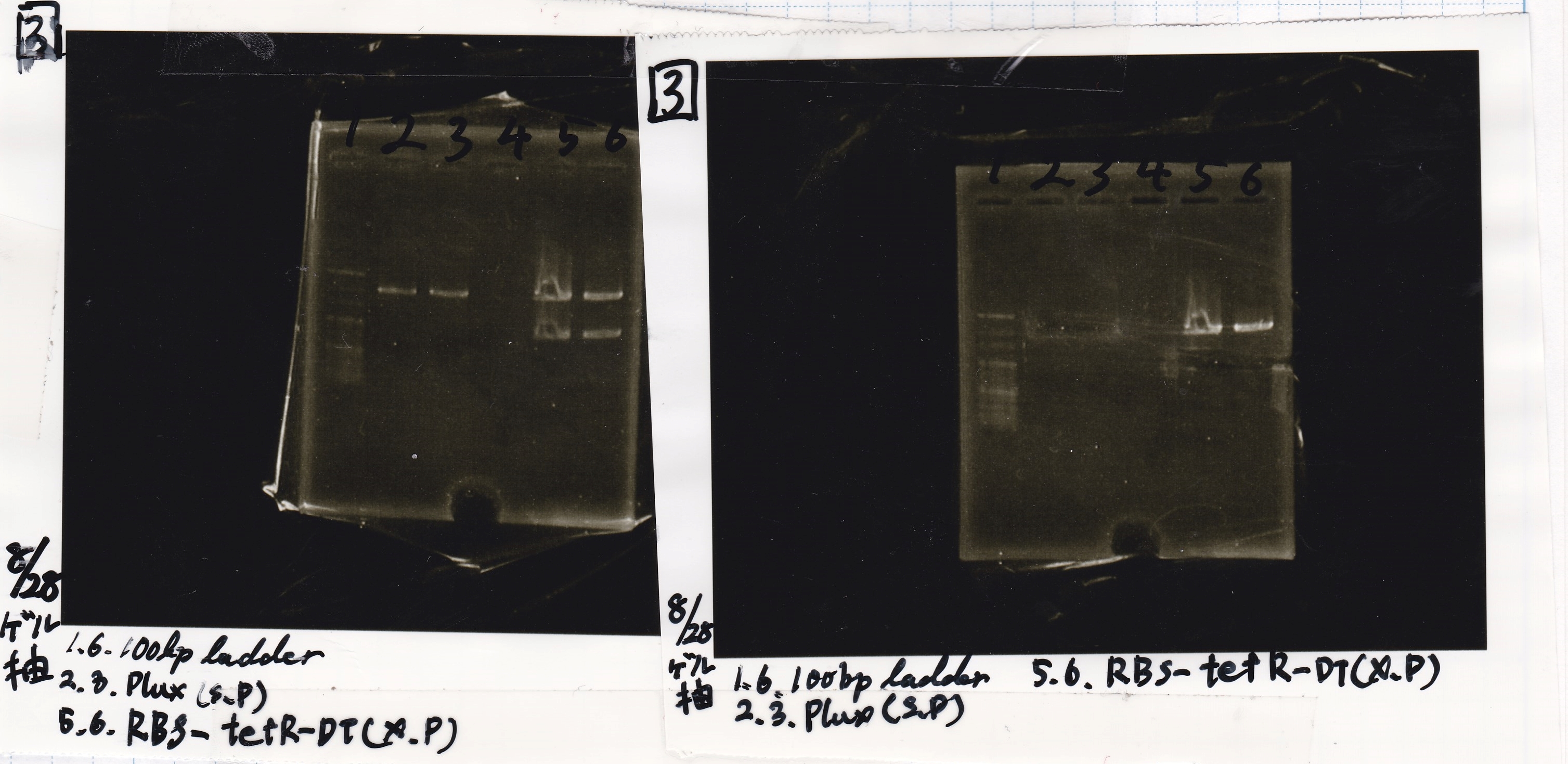
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 2 | 8/20 RBS-lysis1-(1) | EcoRI & SpeI |
| 3 | ||
| 4 | -- | -- |
| 5 | 8/28 RBS-GFP-DT-(2) | XbaI & PstI |
| 6 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 2 | 8/28 Plux | SpeI & PstI |
| 3 | ||
| 4 | -- | -- |
| 5 | 8/20 RBS-tetR-DT-(1) | XbaI & PstI |
| 6 |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Pcon-RBS-GFP-DT (EcoRI&SpeI) | 7.1 | 1.88 | 0.07 |
| Pcon-RBS-luxR-DT (EcoRI&XbaI) | 15.3 | 1.93 | 0.70 |
| Plux (SpeI&PstI) | 4.5 | 1.79 | 0.31 |
| RBS-GFP-DT (XbaI&PstI) | 6.0 | 2.16 | 0.03 |
| RBS-tetR-DT (XbaI&PstI) | 7.6 | 1.91 | 0.38 |
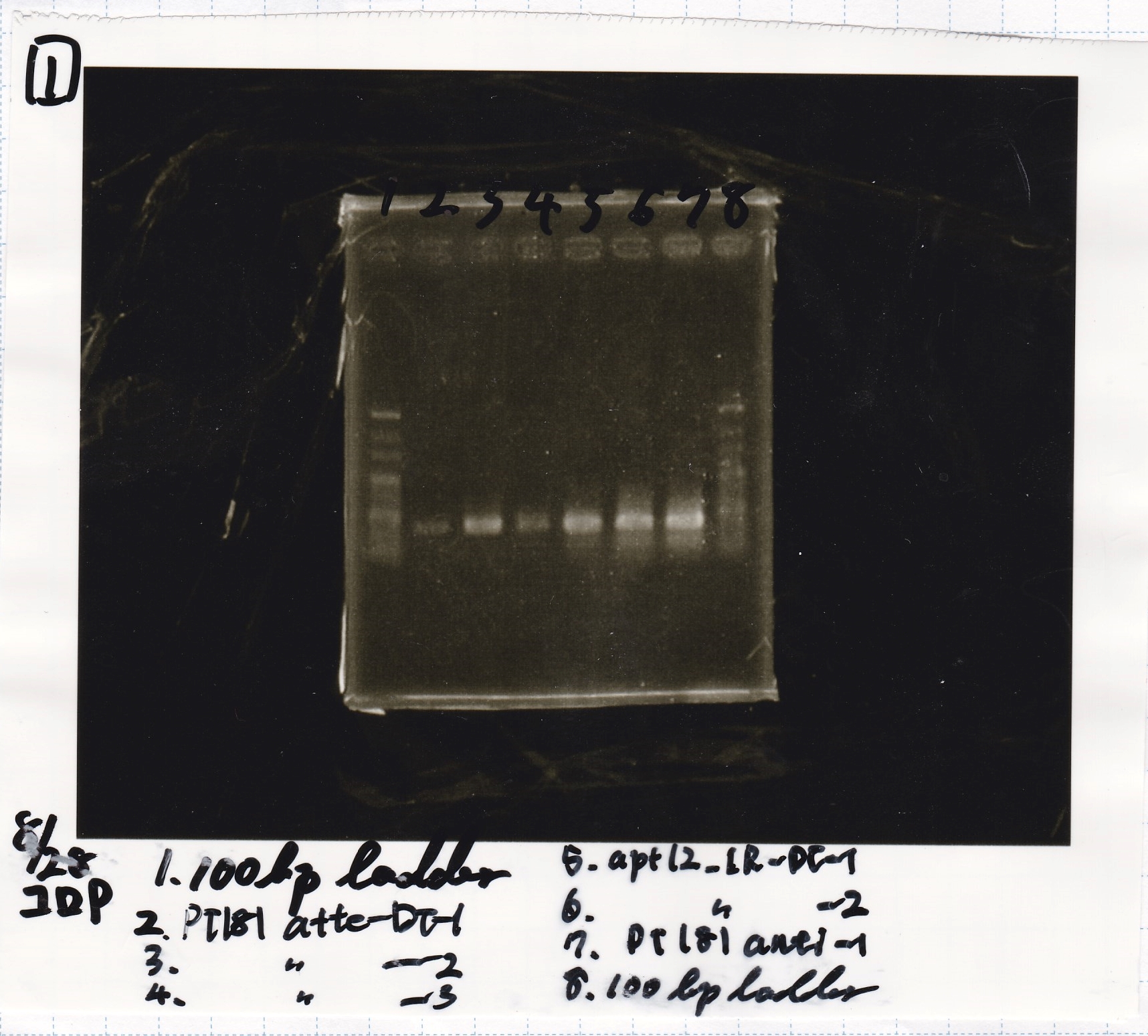
Colony PCR
| Sample | base pair |
|---|---|
| 8/27 pT181 attenuator-DT-1 | 738 |
| 8/27 pT181 attenuator-DT-2 | 738 |
| 8/27 pT181 attenuator-DT-3 | 738 |
| 8/27 TetR-aptamer 12_1R-DT-1 | 521 |
| 8/27 TetR-aptamer 12_1R-DT-2 | 521 |
| 8/27 pT181 antisense-DT-1 | 542 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 30s | 30cycles |
| Sample | base pair |
|---|---|
| 8/27 Pλ-luxI-1 | -- |
| 8/27 Pλ-luxI | -- |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 30min | 3cycles |
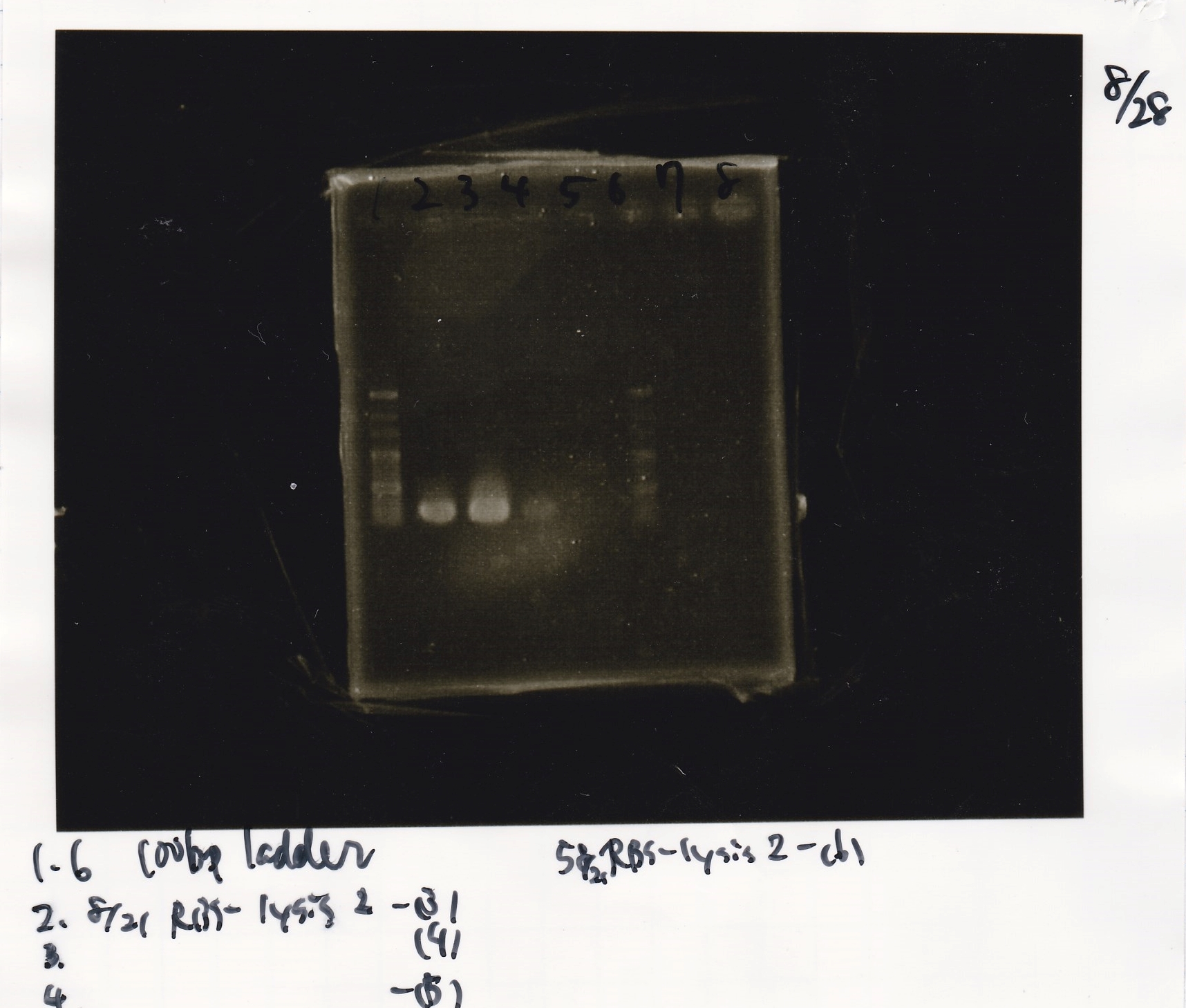
Colony PCR
No name
| Sample | base pair |
|---|---|
| 8/21 RBS-lysis2-9 | 772 |
| 8/21 RBS-lysis2-10 | 772 |
| 8/21 RBS-lysis2-11 | 772 |
| 8/21 RBS-lysis2-12 | 772 |
| 8/21 RBS-lysis2-13 | 772 |
| 8/21 RBS-lysis2-14 | 772 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 48s | 30cycles |
Liquid Culture
| Sample | medium |
|---|---|
| 8/16 Master Plate(CP)-6 DT | Plusgrow medium(+CP) |
| J23100 Master Plate-1 | Plusgrow medium(+Amp) |
| Plac Master Plate-1 | Plusgrow medium(+CP) |
Ligation
| state | Vector | Inserter | ||
|---|---|---|---|---|
| experiment | 8/27 DT (EcoRI & XbaI) | 2.9 | 8/28 RBS-lysis1 (EcoRI & SpeI) | 9.5 |
| experiment | 8/28 Plux (SpeI & PstI) | 2.3 | 8/28 RBS-GFP-DT (XbaI & PstI) | 18 |
| experiment | 8/28 Pcon-RBS-luxR-DT (EcoRI & XbaI) | 3.3 | 8/28 Pcon^RBS-GFP-DT-(1) (EcoRI & SpeI) | 28 |
| experiment | 8/27 DT (EcoRI & XbaI) | 2.7 | 8/24 Spinach (EcoRI & SpeI) | 2.4 |
- Samples were evaporeted used evaporator into about 1 µL.
| sample | MilliQ | Ligation High | total |
|---|---|---|---|
| 1 | 3 | 4 | 9 |
- incubate overnight at 4 °C
Liquid Culture
| Sample | medium |
|---|---|
| 8/26 Pλ-RBS-luxI-DT-1 | Plusgrow medium (+Amp) |
| 8/26 Pλ-RBS-luxI-DT-2 | Plusgrow medium (+Amp) |
- incubate 37°C 10hour
 "
"