Template:Kyoto/Notebook/Aug 31
From 2013.igem.org
(Difference between revisions)
(→Gel Extraction) |
(→Miniprep) |
||
| (6 intermediate revisions not shown) | |||
| Line 22: | Line 22: | ||
|8||--||-- | |8||--||-- | ||
|- | |- | ||
| - | |9||rowspan=2"|pSB1C3(1)||rowspan="2"|XbaI & PstI | + | |9||rowspan="2"|pSB1C3(1)||rowspan="2"|XbaI & PstI |
| + | |- | ||
| + | |10 | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug30 Gel Extraction(N1).jpg]]<br> |
| - | [[File: | + | [[File:Igku Aug30 Gel Extraction(N2-1).jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Lane||DNA||Enzyme | !Lane||DNA||Enzyme | ||
| Line 51: | Line 53: | ||
|11 | |11 | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Aug31 Gel Extraction(N2-2).jpg]]<br> |
| - | [[File: | + | [[File:Igku Aug31 Gel Extraction(N2-3).jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
| Line 69: | Line 71: | ||
|6 | |6 | ||
|} | |} | ||
| + | [[File:Igku Aug31 Gel Extraction(N3-1).jpg]]<br> | ||
| + | [[File:Igku Aug31 Gel Extraction(N3-2).jpg]]<br> | ||
| + | |||
{| class="wikitable" | {| class="wikitable" | ||
!Name||concentration[µg/mL]||260/280||260/230 | !Name||concentration[µg/mL]||260/280||260/230 | ||
| Line 97: | Line 102: | ||
!state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | !state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | ||
|- | |- | ||
| - | |experiment||8/30 pSB1C3(EcoRI&SpeI)||6.6||8/30 tRNA-Spinach(EcoRI&SpeI)||12.5||3.5 | + | |experiment||8/30 pSB1C3 (EcoRI & SpeI)||6.6||8/30 tRNA-Spinach (EcoRI & SpeI)||12.5||3.5 |
|- | |- | ||
| - | |experiment||8/30 pSB1C3(EcoRI&SpeI)||6.6||8/30 pT181 antisense(EcoRI&SpeI)||12.5||3.5 | + | |experiment||8/30 pSB1C3 (EcoRI & SpeI)||6.6||8/30 pT181 antisense (EcoRI & SpeI)||12.5||3.5 |
|- | |- | ||
| - | |experiment||8/30 pSB1C3(XbaI&Pst)||12.5||8/30 pT181 attenuator(XbaI&PstI)||25||3.5 | + | |experiment||8/30 pSB1C3 (XbaI & Pst)||12.5||8/30 pT181 attenuator (XbaI & PstI)||25||3.5 |
|- | |- | ||
| - | |experiment||8/30 pSB1C3(XbaI&Pst)||12.5||8/30 pT181 antisense(XbaI&PstI)||25||3.5 | + | |experiment||8/30 pSB1C3 (XbaI & Pst)||12.5||8/30 pT181 antisense (XbaI & PstI)||25||3.5 |
|- | |- | ||
| - | |experiment||8/20 DT||6.6||8/30 pT181 attenuator(2)||12.5||3.5 | + | |experiment||8/20 DT (EcoRI & XbaI)||6.6||8/30 pT181 attenuator(2) (EcoRI & SpeI)||12.5||3.5 |
|- | |- | ||
| - | |experiment||8/20 DT||6.6||8/30 pT181 antisense||12.5||3.5 | + | |experiment||8/20 DT (EcoRI & XbaI)||6.6||8/30 pT181 antisense (EcoRI & SpeI)||12.5||3.5 |
|- | |- | ||
| - | |experiment||8/27 DT||3.0||8/30 tRNA-Spinach(EcoRI&SpeI)||12.5||3.5 | + | |experiment||8/27 DT (EcoRI & XbaI)||3.0||8/30 tRNA-Spinach (EcoRI & SpeI)||12.5||3.5 |
|- | |- | ||
| - | |experiment||8/20 DT||6.6||8/30 RBS-lysis2||12.5||3.5 | + | |experiment||8/20 DT (EcoRI & XbaI)||6.6||8/30 RBS-lysis2 (EcoRI & SpeI)||12.5||3.5 |
|- | |- | ||
| - | |experiment||8/22 J23100(SpeI&PstI)||--||RBS-tetR-DT||--||3.5 | + | |experiment||8/22 J23100 (SpeI & PstI)||--||RBS-tetR-DT (XbaI & PstI)||--||3.5 |
|- | |- | ||
| - | |experiment||8/22 Plac(SpeI&PstI)||8.0||8/18 RBS- | + | |experiment||8/22 Plac (SpeI & PstI)||8.0||8/18 RBS-lacZα-DT (XbaI & PstI)||10||3.5 |
|} | |} | ||
Samples were evaporeted used evaporator into about 7 µL | Samples were evaporeted used evaporator into about 7 µL | ||
</div> | </div> | ||
| + | |||
===Miniprep=== | ===Miniprep=== | ||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author">Kojima Nakamoto</span> | + | <span class="author">Kojima and Nakamoto</span> |
{|class="wikitable" | {|class="wikitable" | ||
!DNA||concentration[µg/mL]||260/280||260/230 | !DNA||concentration[µg/mL]||260/280||260/230 | ||
|- | |- | ||
| - | |8/30 Plux-RBS-GFP-DT-(1)|| | + | |8/30 Plux-RBS-GFP-DT-(1)||124||1.77||1.87 |
|- | |- | ||
| - | |8/30 Plux-RBS-GFP-DT-(2)|| | + | |8/30 Plux-RBS-GFP-DT-(2)||213||1.75||1.52 |
|- | |- | ||
| - | |8/30 Pbad/araC-RBS-RFP|| | + | |8/30 Pbad/araC-RBS-RFP||427||1.70||1.56 |
|- | |- | ||
|8/30 Pbad/araC-RBS-RFP||670||1.30||0.83 | |8/30 Pbad/araC-RBS-RFP||670||1.30||0.83 | ||
Latest revision as of 03:52, 28 September 2013
Contents |
Aug 31
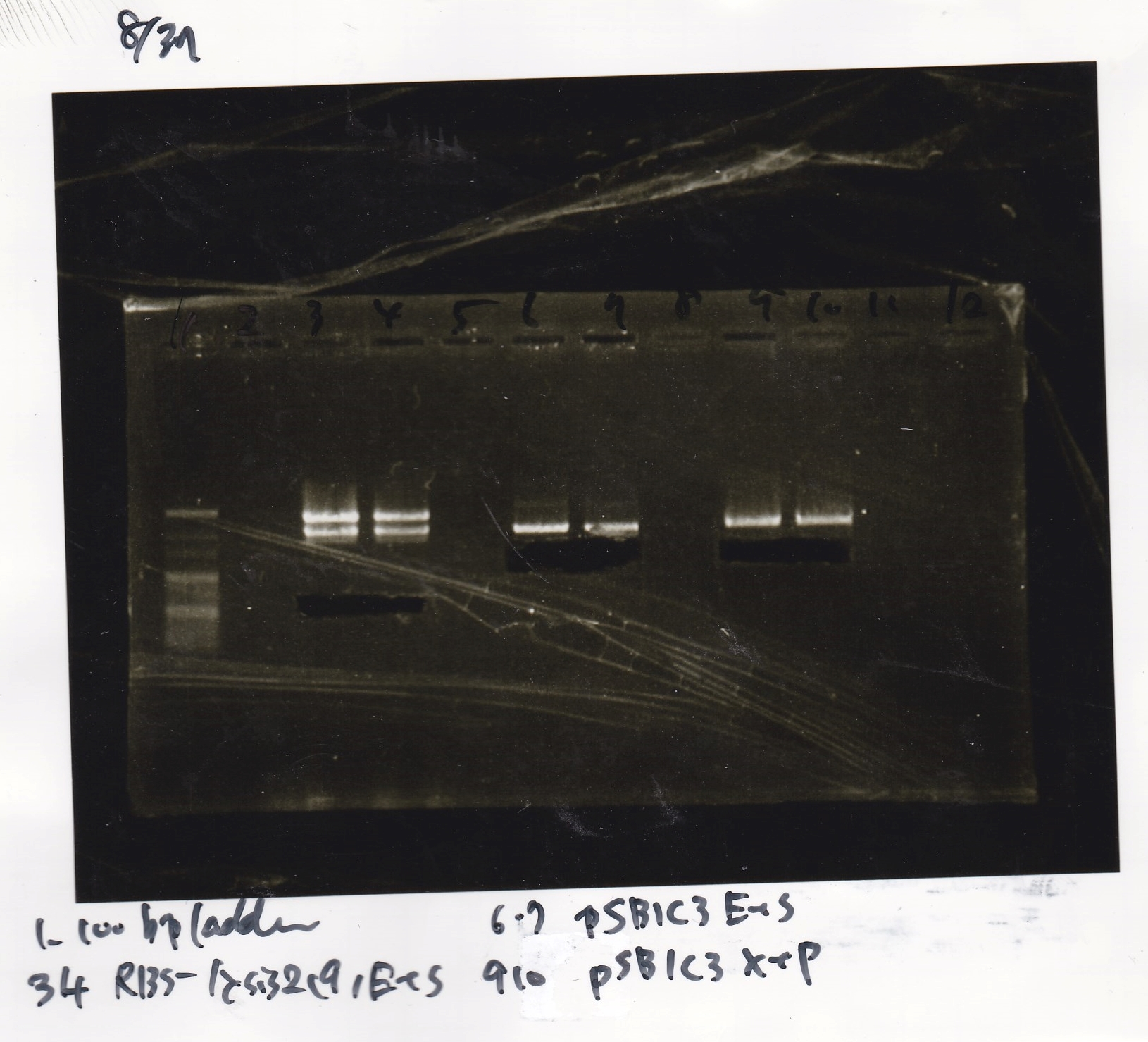
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 2 | -- | -- |
| 3 | RBS-lysis2-(9) | EcoRI & SpeI |
| 4 | ||
| 5 | -- | -- |
| 6 | pSB1C3(1) | EcoRI & SpeI |
| 7 | ||
| 8 | -- | -- |
| 9 | pSB1C3(1) | XbaI & PstI |
| 10 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 2 | tRNA-Spinach | EcoRI & SpeI |
| 3 | ||
| 4 | -- | -- |
| 5 | pT181 attenuator-(2) | EcoRI & SpeI |
| 6 | ||
| 7 | pT181 attenuator-(2) | XbaI & PstI |
| 8 | ||
| 9 | -- | -- |
| 10 | pT181 antisense-(2) | EcoRI & SpeI |
| 11 |
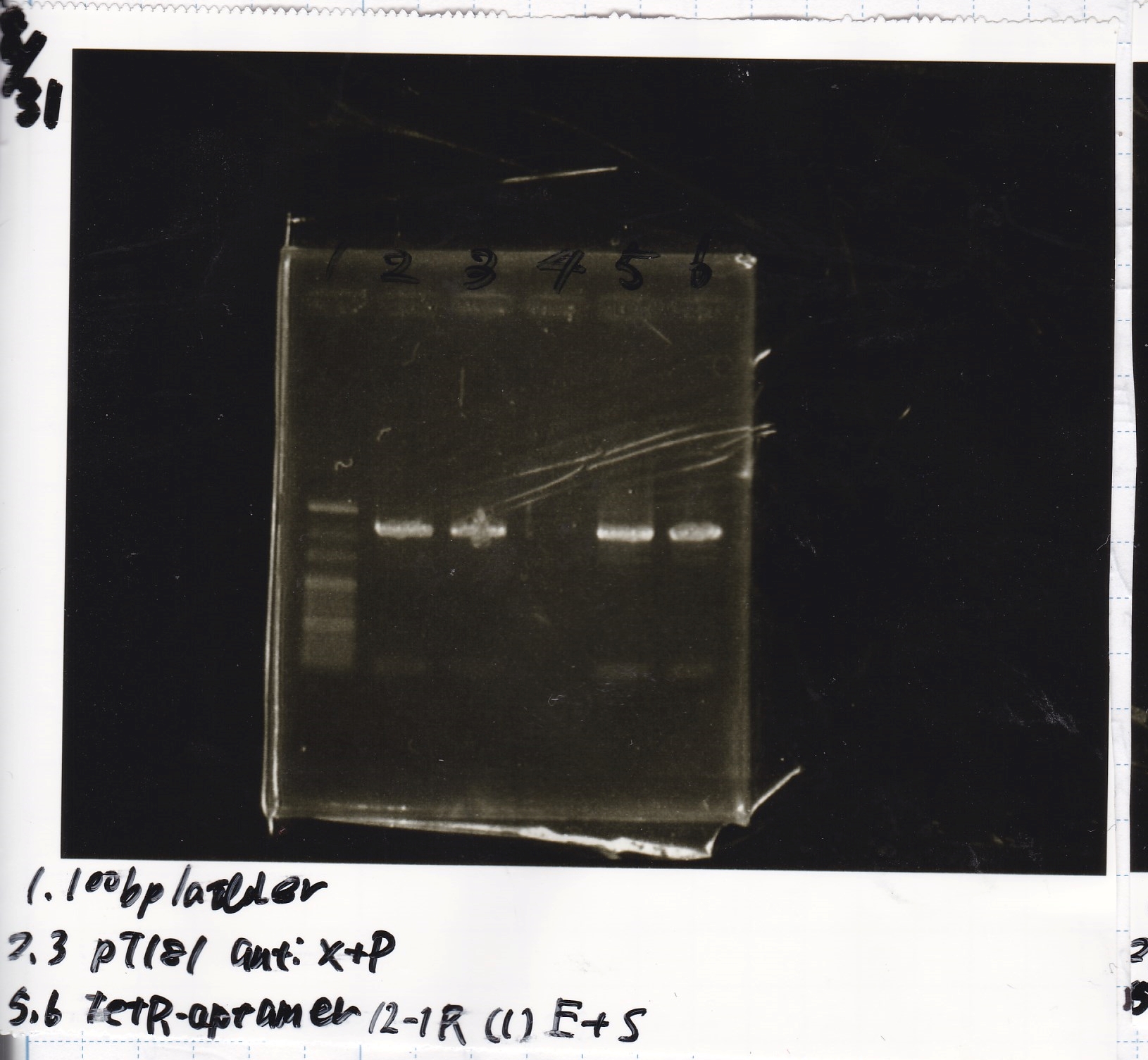
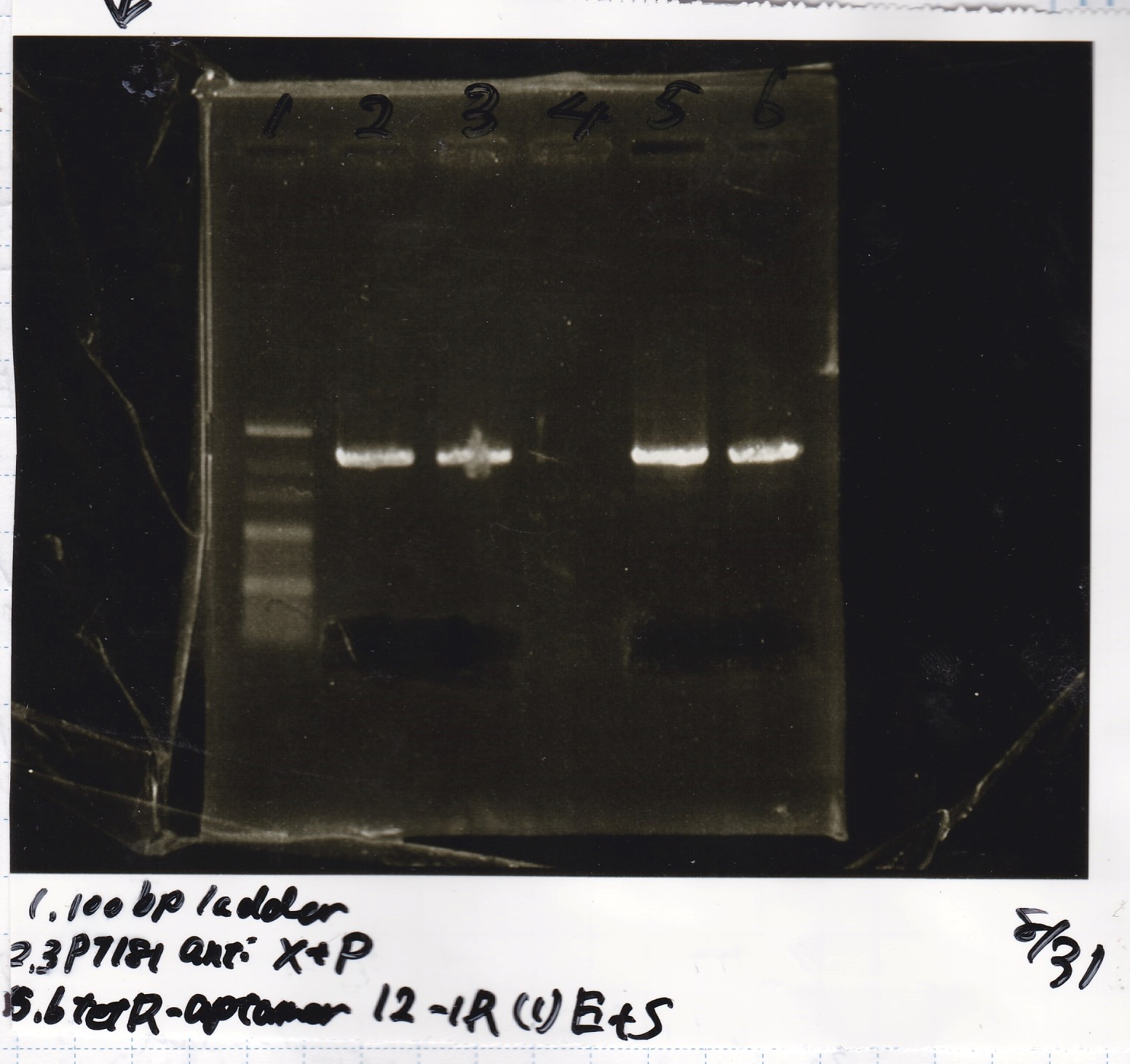
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 2 | pT181 antisense | XbaRI & PstI |
| 3 | ||
| 4 | -- | -- |
| 5 | tetR aptamer 12_1R-(1) | EcoRI & SpeI |
| 6 |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| RBS-lysis2(EcoRI&SpeI) | 4.0 | 2.69 | 0.24 |
| pSB1C3(EcoRI&SpeI) | 6.0 | 2.48 | 0.05 |
| pSB1C3(XbaI&PstI) | -- | -- | -- |
| tRNA-Spinach | -- | -- | -- |
| pT181 attenuator-(2)(EcoRI&SpeI) | -- | -- | -- |
| pT181 anttenuator-(2)(SbaI&PstI) | -- | -- | -- |
| pT181 antisense-(2)(EcoRI&SpeI) | -- | -- | -- |
| pT181 antisense-(2)(XbaI&PstI) | -- | -- | -- |
| tetR aptamer 12_1R(EcoRI&SpeI) | -- | -- | -- |
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | 8/30 pSB1C3 (EcoRI & SpeI) | 6.6 | 8/30 tRNA-Spinach (EcoRI & SpeI) | 12.5 | 3.5 |
| experiment | 8/30 pSB1C3 (EcoRI & SpeI) | 6.6 | 8/30 pT181 antisense (EcoRI & SpeI) | 12.5 | 3.5 |
| experiment | 8/30 pSB1C3 (XbaI & Pst) | 12.5 | 8/30 pT181 attenuator (XbaI & PstI) | 25 | 3.5 |
| experiment | 8/30 pSB1C3 (XbaI & Pst) | 12.5 | 8/30 pT181 antisense (XbaI & PstI) | 25 | 3.5 |
| experiment | 8/20 DT (EcoRI & XbaI) | 6.6 | 8/30 pT181 attenuator(2) (EcoRI & SpeI) | 12.5 | 3.5 |
| experiment | 8/20 DT (EcoRI & XbaI) | 6.6 | 8/30 pT181 antisense (EcoRI & SpeI) | 12.5 | 3.5 |
| experiment | 8/27 DT (EcoRI & XbaI) | 3.0 | 8/30 tRNA-Spinach (EcoRI & SpeI) | 12.5 | 3.5 |
| experiment | 8/20 DT (EcoRI & XbaI) | 6.6 | 8/30 RBS-lysis2 (EcoRI & SpeI) | 12.5 | 3.5 |
| experiment | 8/22 J23100 (SpeI & PstI) | -- | RBS-tetR-DT (XbaI & PstI) | -- | 3.5 |
| experiment | 8/22 Plac (SpeI & PstI) | 8.0 | 8/18 RBS-lacZα-DT (XbaI & PstI) | 10 | 3.5 |
Samples were evaporeted used evaporator into about 7 µL
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 8/30 Plux-RBS-GFP-DT-(1) | 124 | 1.77 | 1.87 |
| 8/30 Plux-RBS-GFP-DT-(2) | 213 | 1.75 | 1.52 |
| 8/30 Pbad/araC-RBS-RFP | 427 | 1.70 | 1.56 |
| 8/30 Pbad/araC-RBS-RFP | 670 | 1.30 | 0.83 |
| 8/30 Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT-(1) | 332 | 1.73 | 2.04 |
| 8/30 Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT-(2) | 478 | 1.74 | 2.09 |
| 8/30 Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT-(3) | 146 | 1.77 | 2.11 |
| 8/30 Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT-(4) | 454 | 1.75 | 2.19 |
 "
"