Template:Kyoto/Notebook/Sep 7
From 2013.igem.org
(Difference between revisions)
(Created page with "==Sep 7==") |
(→Electrophoresis) |
||
| (31 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
==Sep 7== | ==Sep 7== | ||
| + | ===Miniprep=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {|class="wikitable" | ||
| + | !DNA||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |RBS-lysis1-DT||195.3 || || | ||
| + | |- | ||
| + | |RBS-lysis3-DT||255.5 ||1.91 ||2.04 | ||
| + | |- | ||
| + | |Ptet-pT18||183.6 ||1.85 ||1.48 | ||
| + | |- | ||
| + | |Pcon-RBS-tetR-DT||134.3 ||1.94 ||2.20 | ||
| + | |- | ||
| + | |Ptet-RBS-lacZα-DT||67.7 ||1.92 ||1.79 | ||
| + | |- | ||
| + | |Pcon-pT181 attenuator||232.3 ||1.95 ||1.90 | ||
| + | |- | ||
| + | |pSB4K5||237.0 ||1.61 ||1.50 | ||
| + | |- | ||
| + | |pT181 antisense(pSB1C3)||256.4 ||1.63 ||1.78 | ||
| + | |- | ||
| + | |apt12_1R(pSB1C3)||230.0 ||1.98 ||2.28 | ||
| + | |} | ||
| + | </div> | ||
| + | ===Electrophoresis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
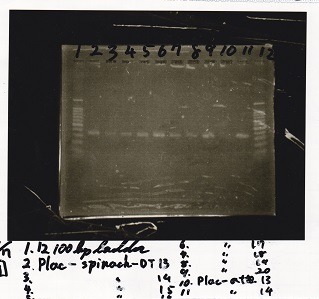
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||100bp ladder | ||
| + | |- | ||
| + | |2||Plac-Spinach-DT -5 | ||
| + | |- | ||
| + | |3||Plac-Spinach-DT -6 | ||
| + | |- | ||
| + | |4||Plac-Spinach-DT -7 | ||
| + | |- | ||
| + | |5||Plac-Spinach-DT -8 | ||
| + | |- | ||
| + | |6||Plac-Spinach-DT -9 | ||
| + | |- | ||
| + | |7||Plac-Spinach-DT -10 | ||
| + | |- | ||
| + | |8||100bp ladder | ||
| + | |- | ||
| + | |1||100bp ladder | ||
| + | |- | ||
| + | |2||Plac-Spinach-DT -11 | ||
| + | |- | ||
| + | |3||Plac-Spinach-DT -12 | ||
| + | |- | ||
| + | |4||Pcon-antisense -5 | ||
| + | |- | ||
| + | |5||Pcon-antisense -6 | ||
| + | |- | ||
| + | |6||Pcon-antisense -7 | ||
| + | |- | ||
| + | |7||Pcon-antisense -8 | ||
| + | |- | ||
| + | |8||100bp ladder | ||
| + | |} | ||
| + | [[File:Igku_Sep7electrophoresis1.jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||100bp ladder | ||
| + | |- | ||
| + | |2||Pcon-antisense -9 | ||
| + | |- | ||
| + | |3||Pcon-antisense -10 | ||
| + | |- | ||
| + | |4||Pcon-antisense -11 | ||
| + | |- | ||
| + | |5||Pcon-antisense -12 | ||
| + | |- | ||
| + | |6||Plac-attenuator -5 | ||
| + | |- | ||
| + | |7||Plac-attenuator -6 | ||
| + | |- | ||
| + | |8||100bp ladder | ||
| + | |- | ||
| + | |1||100bp ladder | ||
| + | |- | ||
| + | |2||Plac-attenuator -7 | ||
| + | |- | ||
| + | |3||Plac-attenuator -8 | ||
| + | |- | ||
| + | |4||Plac-attenuator -9 | ||
| + | |- | ||
| + | |5||Plac-attenuator -10 | ||
| + | |- | ||
| + | |6||Plac-attenuator -11 | ||
| + | |- | ||
| + | |7||Plac-attenuator -12 | ||
| + | |- | ||
| + | |8||100bp ladder | ||
| + | |} | ||
| + | [[File:Igku_Sep7electrophoresis2.jpg]]<br> | ||
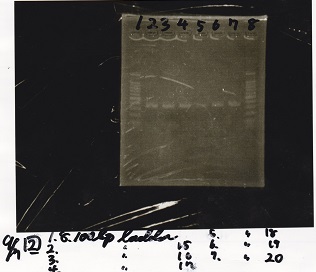
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||100bp ladder | ||
| + | |- | ||
| + | |2||Plac-pT181 antisense | ||
| + | |} | ||
| + | [[File:Igku_Sep7electrophoresis3.jpg]]<br> | ||
| + | </div> | ||
| + | |||
| + | ===Colony PCR=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample | ||
| + | |- | ||
| + | |Plac-spinach-DT -13~20 | ||
| + | |- | ||
| + | |Plac-pT181 attenuator -13~20 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94 °C||94 °C||55 °C||68°C||-- | ||
| + | |- | ||
| + | |5 min||30 s||30 s||42 sec||30 cycles | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Ligation=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No Name</span> | ||
| + | {| class="wikitable" | ||
| + | !state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | ||
| + | |- | ||
| + | |experiment||9/6 Plux (SpeI&PstI)||2 µL||9/7 RBS-lysis2-DT (EcoRI&XbaI)|| µL||3.5 µL | ||
| + | |- | ||
| + | |experiment||9/6 Plac (SpeI&PstI)|| µL||9/7 RBS-lysis2-DT (EcoRI&XbaI)|| µL||3.5 µL | ||
| + | |- | ||
| + | |experiment||9/6 Pcon-RBS-luxR-DT (EcoRI&XbaI)||1.9 µL||9/6 Plux-RBS-GFP-DT(SpeI&PstI)||2.2 µL||3.5 µL | ||
| + | |- | ||
| + | |experiment||9/6 Pbed/araC (SpeI&PstI)||1.6 µL||9/5 RBS-luxI-DT (EcoRI&XbaI)||16 µL||3.5 µL | ||
| + | |- | ||
| + | |experiment||9/6 Plac (SpeI&PstI)||1.5 µL||9/5 pT181 antisense (EcoRI&XbaI)||2.0 µL||3.5 µL | ||
| + | |- | ||
| + | |experiment||9/6 Pcon (SpeI&PstI)||3.0 µL||9/5 RBS-luxI-DT (EcoRI&XbaI)||10 µL||3.5 µL | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Electrophoresis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto</span> | ||
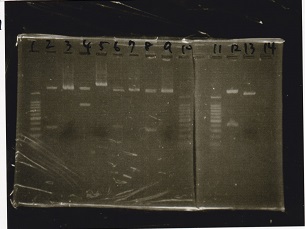
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||100bp ladder | ||
| + | |- | ||
| + | |2||Plac-Spinach-DT -13 | ||
| + | |- | ||
| + | |3||Plac-Spinach-DT -14 | ||
| + | |- | ||
| + | |4||Plac-Spinach-DT -15 | ||
| + | |- | ||
| + | |5||Plac-Spinach-DT -16 | ||
| + | |- | ||
| + | |6||Plac-Spinach-DT -17 | ||
| + | |- | ||
| + | |7||Plac-Spinach-DT -18 | ||
| + | |- | ||
| + | |8||Plac-Spinach-DT -19 | ||
| + | |- | ||
| + | |9||Plac-Spinach-DT -20 | ||
| + | |- | ||
| + | |10||Plac-pT181 attenuator -13 | ||
| + | |- | ||
| + | |11||Plac-pT181 attenuator -14 | ||
| + | |- | ||
| + | |12||100bp ladder | ||
| + | |} | ||
| + | [[File:igku_974.jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||100bp ladder | ||
| + | |- | ||
| + | |2||Plac-pT181 attenuator -15 | ||
| + | |- | ||
| + | |3||Plac-pT181 attenuator -16 | ||
| + | |- | ||
| + | |4||Plac-pT181 attenuator -17 | ||
| + | |- | ||
| + | |5||Plac-pT181 attenuator -18 | ||
| + | |- | ||
| + | |6||Plac-pT181 attenuator -19 | ||
| + | |- | ||
| + | |7||Plac-pT181 attenuator -20 | ||
| + | |- | ||
| + | |8||100bp ladder | ||
| + | |} | ||
| + | [[File:igku_975.jpg]]<br> | ||
| + | </div> | ||
| + | |||
| + | ===Transformation=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Tatsui</span> | ||
| + | {| class="wikitable" | ||
| + | !Name||Sample||Competent Cells||Total||Plate | ||
| + | |- | ||
| + | |Pcon-RBS-luxR-DT+Plux-RBS-GFP-DT||2µL||20µL||22µL|| | ||
| + | |- | ||
| + | |Pbed/araC+RBS-luxI-DT||2µL ||20µL ||22µL || | ||
| + | |- | ||
| + | |Plac+pT181 antisense ||2µL ||20µL ||22µL || | ||
| + | |- | ||
| + | |Pcon+RBS-luxI-DT ||2µL ||20µL ||22µL || | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Restriction Enzyme Digestion=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No Name</span> | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||XbaI||PstI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |9/7 RBS-lysis1-DT(XbaI & PstI)||10 µL||1 µL||1 µL||3 µL||3 µL||12 µL||30 µL | ||
| + | |- | ||
| + | |NC||0.5 µL||0 µL||0 µL||1 µL||1 µL||7.5 µL||10 µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||XbaI||PstI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |9/7 RBS-lysis3-DT (XbaI & PstI)||7.8 µL||1 µL||1 µL||3 µL||3 µL||14.2 µL||30 µL | ||
| + | |- | ||
| + | |NC||0.4 µL||0 µL||0 µL||1 µL||1 µL||7.6 µL||10 µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||SpeI||PstI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |9/5 Plac (SpeI & PstI)||6.0 µL||1 µL||1 µL||3 µL||3 µL||16.0 µL||30 µL | ||
| + | |- | ||
| + | |NC||0.3 µL||0 µL||0 µL||1 µL||1 µL||7.7 µL||10 µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||EcorI||SpeI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |9/7 pSB4K5 (EcoRI & SpeI)||8.4 µL||1 µL||1 µL||3 µL||3 µL||16.0 µL||30 µL | ||
| + | |- | ||
| + | |NC||0.4 µL||0 µL||0 µL||1 µL||1 µL||7.7 µL||10 µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||restricion enzyme||restriction enzyme||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |Pcon-pT181 attenuator (SpeI & PstI)||8.6 µL||1 µL||1 µL||3 µL||3 µL||13.4 µL||30 µL | ||
| + | |- | ||
| + | |Pcon-pT181 attenuator (EcorI & SpeI)||8.6 µL||1 µL||1 µL||3 µL||3 µL||13.4 µL||30 µL | ||
| + | |- | ||
| + | |NC||0.4 µL||0 µL||0 µL||1 µL||1 µL||7.6 µL||10 µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||XbaI||PstI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |9/5 Spinach-DT (XbaI & PstI)||5.4 µL||1 µL||1 µL||3 µL||3 µL||16.6 µL||30 µL | ||
| + | |- | ||
| + | |NC||0.3 µL||0 µL||0 µL||1 µL||1 µL||7.7 µL||10 µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||SpeI||PstI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |Ptet-anticodone (SpeI & PstI)||11 µL||1 µL||1 µL||3 µL||3 µL||11 µL||30 µL | ||
| + | |- | ||
| + | |NC||0.5 µL||0 µL||0 µL||1 µL||1 µL||7.5 µL||10 µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||XbaI||PstI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |9/6 RBS-lacZα-DT (XbaI & PstI)||11 µL||1 µL||1 µL||3 µL||3 µL||11 µL||30 µL | ||
| + | |- | ||
| + | |NC||0.5 µL||0 µL||0 µL||1 µL||1 µL||7.5 µL||10 µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||EcoRI||SpeI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |pSB1C3 (1) (EcoRI & SpeI)||6.6 µL||1 µL||1 µL||3 µL||3 µL||15.4 µL||30 µL | ||
| + | |- | ||
| + | |NC||0.3 µL||0 µL||0 µL||1 µL||1 µL||7.7 µL||10 µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||XbaI||PstI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |pSB1C3 (3) (XbaI & PstI)||6.6 µL||1 µL||1 µL||3 µL||3 µL||15.4 µL||30 µL | ||
| + | |- | ||
| + | |NC||0.3 µL||0 µL||0 µL||1 µL||1 µL||7.7 µL||10 µL | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Electrophoresis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||100bp ladder||--||-- | ||
| + | |- | ||
| + | |2||RBS-lysis1-DT (9/7)||XbaI||PstI | ||
| + | |- | ||
| + | |3||RBS-lysis1-DT (9/7)||--||-- | ||
| + | |- | ||
| + | |4||RBS-lysis3-DT (9/7)||XbaI||PstI | ||
| + | |- | ||
| + | |5||RBS-lysis3-DT (9/7)||--||-- | ||
| + | |- | ||
| + | |6||Pcon-pT181 attenuator||EcoRI||SpeI | ||
| + | |- | ||
| + | |7||Pcon-pT181 attenuator||--||-- | ||
| + | |- | ||
| + | |8||9/5 Spinach-DT||XbaI||PstI | ||
| + | |- | ||
| + | |9||9/5 Spinach-DT||--||-- | ||
| + | |- | ||
| + | |10||100bp ladder||--||-- | ||
| + | |- | ||
| + | |11||100bp ladder||--||-- | ||
| + | |- | ||
| + | |12||RBS-lacZα-DT||XbaI||PstI | ||
| + | |- | ||
| + | |13||RBS-lacZα-DT||--||-- | ||
| + | |} | ||
| + | [[File:igku_971.jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||100bp ladder||--||-- | ||
| + | |- | ||
| + | |2||Ptet-antisense||SpeI||PstI | ||
| + | |- | ||
| + | |3||Plac (NC)||--||-- | ||
| + | |- | ||
| + | |4||pSB4K5||EcoRI||SpeI | ||
| + | |- | ||
| + | |5||pSB4K5||--||-- | ||
| + | |- | ||
| + | |6||Pcon-pT181 attenuator||SpeI||PstI | ||
| + | |- | ||
| + | |7||1kbp ladder||--||-- | ||
| + | |} | ||
| + | [[File:igku_972.jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||100bp ladder||--||-- | ||
| + | |- | ||
| + | |2||Plac||SpeI||PstI | ||
| + | |- | ||
| + | |3||Ptet-antisense||--||-- | ||
| + | |- | ||
| + | |4||pSB1C3||EcoRI||SpeI | ||
| + | |- | ||
| + | |5||pSB1C3||--||-- | ||
| + | |- | ||
| + | |6||pSB1C3||XbaI||PstI | ||
| + | |- | ||
| + | |7||pSB1C3||--||-- | ||
| + | |- | ||
| + | |8||1kbp ladder||--||-- | ||
| + | |} | ||
| + | [[File:igku_973.jpg]]<br> | ||
| + | </div> | ||
| + | |||
| + | ===Liquid Culture=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||medium | ||
| + | |- | ||
| + | |entA- strain||SOB medium (+Kan) | ||
| + | |- | ||
| + | |9/15 Pcon-pT181 antisense||Plusgrow (+Amp) | ||
| + | |} | ||
| + | </div> | ||
Latest revision as of 02:13, 27 September 2013
Contents |
Sep 7
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| RBS-lysis1-DT | 195.3 | ||
| RBS-lysis3-DT | 255.5 | 1.91 | 2.04 |
| Ptet-pT18 | 183.6 | 1.85 | 1.48 |
| Pcon-RBS-tetR-DT | 134.3 | 1.94 | 2.20 |
| Ptet-RBS-lacZα-DT | 67.7 | 1.92 | 1.79 |
| Pcon-pT181 attenuator | 232.3 | 1.95 | 1.90 |
| pSB4K5 | 237.0 | 1.61 | 1.50 |
| pT181 antisense(pSB1C3) | 256.4 | 1.63 | 1.78 |
| apt12_1R(pSB1C3) | 230.0 | 1.98 | 2.28 |
Electrophoresis
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | Plac-Spinach-DT -5 |
| 3 | Plac-Spinach-DT -6 |
| 4 | Plac-Spinach-DT -7 |
| 5 | Plac-Spinach-DT -8 |
| 6 | Plac-Spinach-DT -9 |
| 7 | Plac-Spinach-DT -10 |
| 8 | 100bp ladder |
| 1 | 100bp ladder |
| 2 | Plac-Spinach-DT -11 |
| 3 | Plac-Spinach-DT -12 |
| 4 | Pcon-antisense -5 |
| 5 | Pcon-antisense -6 |
| 6 | Pcon-antisense -7 |
| 7 | Pcon-antisense -8 |
| 8 | 100bp ladder |
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | Pcon-antisense -9 |
| 3 | Pcon-antisense -10 |
| 4 | Pcon-antisense -11 |
| 5 | Pcon-antisense -12 |
| 6 | Plac-attenuator -5 |
| 7 | Plac-attenuator -6 |
| 8 | 100bp ladder |
| 1 | 100bp ladder |
| 2 | Plac-attenuator -7 |
| 3 | Plac-attenuator -8 |
| 4 | Plac-attenuator -9 |
| 5 | Plac-attenuator -10 |
| 6 | Plac-attenuator -11 |
| 7 | Plac-attenuator -12 |
| 8 | 100bp ladder |
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | Plac-pT181 antisense |
Colony PCR
| Sample |
|---|
| Plac-spinach-DT -13~20 |
| Plac-pT181 attenuator -13~20 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94 °C | 94 °C | 55 °C | 68°C | -- |
| 5 min | 30 s | 30 s | 42 sec | 30 cycles |
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | 9/6 Plux (SpeI&PstI) | 2 µL | 9/7 RBS-lysis2-DT (EcoRI&XbaI) | µL | 3.5 µL |
| experiment | 9/6 Plac (SpeI&PstI) | µL | 9/7 RBS-lysis2-DT (EcoRI&XbaI) | µL | 3.5 µL |
| experiment | 9/6 Pcon-RBS-luxR-DT (EcoRI&XbaI) | 1.9 µL | 9/6 Plux-RBS-GFP-DT(SpeI&PstI) | 2.2 µL | 3.5 µL |
| experiment | 9/6 Pbed/araC (SpeI&PstI) | 1.6 µL | 9/5 RBS-luxI-DT (EcoRI&XbaI) | 16 µL | 3.5 µL |
| experiment | 9/6 Plac (SpeI&PstI) | 1.5 µL | 9/5 pT181 antisense (EcoRI&XbaI) | 2.0 µL | 3.5 µL |
| experiment | 9/6 Pcon (SpeI&PstI) | 3.0 µL | 9/5 RBS-luxI-DT (EcoRI&XbaI) | 10 µL | 3.5 µL |
Electrophoresis
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | Plac-Spinach-DT -13 |
| 3 | Plac-Spinach-DT -14 |
| 4 | Plac-Spinach-DT -15 |
| 5 | Plac-Spinach-DT -16 |
| 6 | Plac-Spinach-DT -17 |
| 7 | Plac-Spinach-DT -18 |
| 8 | Plac-Spinach-DT -19 |
| 9 | Plac-Spinach-DT -20 |
| 10 | Plac-pT181 attenuator -13 |
| 11 | Plac-pT181 attenuator -14 |
| 12 | 100bp ladder |
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | Plac-pT181 attenuator -15 |
| 3 | Plac-pT181 attenuator -16 |
| 4 | Plac-pT181 attenuator -17 |
| 5 | Plac-pT181 attenuator -18 |
| 6 | Plac-pT181 attenuator -19 |
| 7 | Plac-pT181 attenuator -20 |
| 8 | 100bp ladder |
Transformation
| Name | Sample | Competent Cells | Total | Plate |
|---|---|---|---|---|
| Pcon-RBS-luxR-DT+Plux-RBS-GFP-DT | 2µL | 20µL | 22µL | |
| Pbed/araC+RBS-luxI-DT | 2µL | 20µL | 22µL | |
| Plac+pT181 antisense | 2µL | 20µL | 22µL | |
| Pcon+RBS-luxI-DT | 2µL | 20µL | 22µL |
Restriction Enzyme Digestion
| DNA | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 9/7 RBS-lysis1-DT(XbaI & PstI) | 10 µL | 1 µL | 1 µL | 3 µL | 3 µL | 12 µL | 30 µL |
| NC | 0.5 µL | 0 µL | 0 µL | 1 µL | 1 µL | 7.5 µL | 10 µL |
| DNA | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 9/7 RBS-lysis3-DT (XbaI & PstI) | 7.8 µL | 1 µL | 1 µL | 3 µL | 3 µL | 14.2 µL | 30 µL |
| NC | 0.4 µL | 0 µL | 0 µL | 1 µL | 1 µL | 7.6 µL | 10 µL |
| DNA | SpeI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 9/5 Plac (SpeI & PstI) | 6.0 µL | 1 µL | 1 µL | 3 µL | 3 µL | 16.0 µL | 30 µL |
| NC | 0.3 µL | 0 µL | 0 µL | 1 µL | 1 µL | 7.7 µL | 10 µL |
| DNA | EcorI | SpeI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 9/7 pSB4K5 (EcoRI & SpeI) | 8.4 µL | 1 µL | 1 µL | 3 µL | 3 µL | 16.0 µL | 30 µL |
| NC | 0.4 µL | 0 µL | 0 µL | 1 µL | 1 µL | 7.7 µL | 10 µL |
| DNA | restricion enzyme | restriction enzyme | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| Pcon-pT181 attenuator (SpeI & PstI) | 8.6 µL | 1 µL | 1 µL | 3 µL | 3 µL | 13.4 µL | 30 µL |
| Pcon-pT181 attenuator (EcorI & SpeI) | 8.6 µL | 1 µL | 1 µL | 3 µL | 3 µL | 13.4 µL | 30 µL |
| NC | 0.4 µL | 0 µL | 0 µL | 1 µL | 1 µL | 7.6 µL | 10 µL |
| DNA | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 9/5 Spinach-DT (XbaI & PstI) | 5.4 µL | 1 µL | 1 µL | 3 µL | 3 µL | 16.6 µL | 30 µL |
| NC | 0.3 µL | 0 µL | 0 µL | 1 µL | 1 µL | 7.7 µL | 10 µL |
| DNA | SpeI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| Ptet-anticodone (SpeI & PstI) | 11 µL | 1 µL | 1 µL | 3 µL | 3 µL | 11 µL | 30 µL |
| NC | 0.5 µL | 0 µL | 0 µL | 1 µL | 1 µL | 7.5 µL | 10 µL |
| DNA | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 9/6 RBS-lacZα-DT (XbaI & PstI) | 11 µL | 1 µL | 1 µL | 3 µL | 3 µL | 11 µL | 30 µL |
| NC | 0.5 µL | 0 µL | 0 µL | 1 µL | 1 µL | 7.5 µL | 10 µL |
| DNA | EcoRI | SpeI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| pSB1C3 (1) (EcoRI & SpeI) | 6.6 µL | 1 µL | 1 µL | 3 µL | 3 µL | 15.4 µL | 30 µL |
| NC | 0.3 µL | 0 µL | 0 µL | 1 µL | 1 µL | 7.7 µL | 10 µL |
| DNA | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| pSB1C3 (3) (XbaI & PstI) | 6.6 µL | 1 µL | 1 µL | 3 µL | 3 µL | 15.4 µL | 30 µL |
| NC | 0.3 µL | 0 µL | 0 µL | 1 µL | 1 µL | 7.7 µL | 10 µL |
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | RBS-lysis1-DT (9/7) | XbaI | PstI |
| 3 | RBS-lysis1-DT (9/7) | -- | -- |
| 4 | RBS-lysis3-DT (9/7) | XbaI | PstI |
| 5 | RBS-lysis3-DT (9/7) | -- | -- |
| 6 | Pcon-pT181 attenuator | EcoRI | SpeI |
| 7 | Pcon-pT181 attenuator | -- | -- |
| 8 | 9/5 Spinach-DT | XbaI | PstI |
| 9 | 9/5 Spinach-DT | -- | -- |
| 10 | 100bp ladder | -- | -- |
| 11 | 100bp ladder | -- | -- |
| 12 | RBS-lacZα-DT | XbaI | PstI |
| 13 | RBS-lacZα-DT | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | Ptet-antisense | SpeI | PstI |
| 3 | Plac (NC) | -- | -- |
| 4 | pSB4K5 | EcoRI | SpeI |
| 5 | pSB4K5 | -- | -- |
| 6 | Pcon-pT181 attenuator | SpeI | PstI |
| 7 | 1kbp ladder | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | Plac | SpeI | PstI |
| 3 | Ptet-antisense | -- | -- |
| 4 | pSB1C3 | EcoRI | SpeI |
| 5 | pSB1C3 | -- | -- |
| 6 | pSB1C3 | XbaI | PstI |
| 7 | pSB1C3 | -- | -- |
| 8 | 1kbp ladder | -- | -- |
Liquid Culture
| Sample | medium |
|---|---|
| entA- strain | SOB medium (+Kan) |
| 9/15 Pcon-pT181 antisense | Plusgrow (+Amp) |
 "
"