Template:Kyoto/Notebook/Aug 16
From 2013.igem.org
(Difference between revisions)
(→Colony PCR) |
(→Transformation) |
||
| (25 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
===Colony PCR=== | ===Colony PCR=== | ||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">Tatsui and Ashida</span> |
{| class="wikitable" | {| class="wikitable" | ||
!Sample||base pair | !Sample||base pair | ||
|- | |- | ||
| - | |( | + | |8/15 BBa_I13504(RBS-GFP-DT)(1)||1174 |
| + | |- | ||
| + | |8/15 BBa_I13504(RBS-GFP-DT)(2)||1174 | ||
| + | |- | ||
| + | |8/15 BBa_I0462(RBS-luxR-DT)(1)||1174 | ||
| + | |- | ||
| + | |8/15 BBa_I0462(RBS-luxR-DT)(2)||1174 | ||
| + | |- | ||
| + | |8/15 BBa_K748002(lysis3)||1053 | ||
| + | |- | ||
| + | |NC||-- | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
!PreDenature||Denature||Annealing||Extension||cycle | !PreDenature||Denature||Annealing||Extension||cycle | ||
|- | |- | ||
| - | | | + | |94°C||94°C||55°C||68°C||-- |
|- | |- | ||
| - | | | + | |5min||30s||30s||1min||30cycles |
|} | |} | ||
</div> | </div> | ||
| - | + | ||
| - | + | ||
{| class="wikitable" | {| class="wikitable" | ||
!Sample||base pair | !Sample||base pair | ||
|- | |- | ||
| - | |( | + | |8/15 BBa_R0061(Plux)||344 |
| + | |- | ||
| + | |8/15 BBa_K117000(lysis1)(1)||456 | ||
| + | |- | ||
| + | |8/15 BBa_K117000(lysis1)(2)||456 | ||
| + | |- | ||
| + | |8/15 BBa_B0015(DT)||443 | ||
| + | |- | ||
| + | |8/15 BBa_I732020(RBS-lacZα-DT)||703 | ||
| + | |- | ||
| + | |NC||-- | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
!PreDenature||Denature||Annealing||Extension||cycle | !PreDenature||Denature||Annealing||Extension||cycle | ||
|- | |- | ||
| - | | | + | |94°C||94°C||55°C||68°C||-- |
|- | |- | ||
| - | | | + | |5min||30s||30s||40s||30cycles |
|} | |} | ||
| + | |||
| + | ===Electrophoresis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
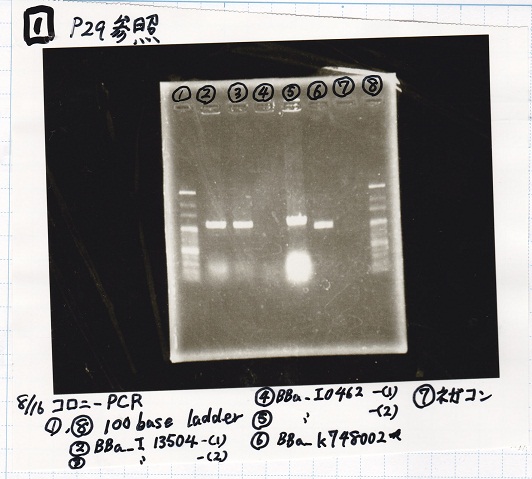
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||100bp ladder | ||
| + | |- | ||
| + | |2||BBa_I13504(RBS-GFP-DT) -(1) | ||
| + | |- | ||
| + | |3||BBa_I13504(RBS-GFP-DT) -(2) | ||
| + | |- | ||
| + | |4||BBa_I0462(RBS-luxR-DT) -(1) | ||
| + | |- | ||
| + | |5||BBa_I0462(RBS-luxR-DT) -(2) | ||
| + | |- | ||
| + | |6||BBa_K748002(lysis3) | ||
| + | |- | ||
| + | |7||NC | ||
| + | |- | ||
| + | |8||100bp ladder | ||
| + | |} | ||
| + | [[File:igku_8161.jpg]]<br> | ||
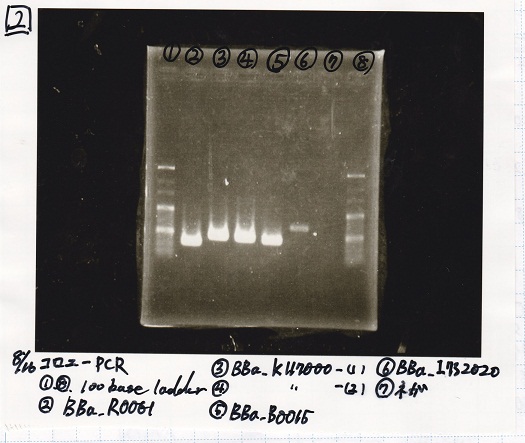
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||100bp ladder | ||
| + | |- | ||
| + | |2||BBa_R0061(Plux) | ||
| + | |- | ||
| + | |3||BBa_K117000(lysis1) -(1) | ||
| + | |- | ||
| + | |4||BBa_K117000(lysis1) -(1) | ||
| + | |- | ||
| + | |5||BBa_B0015(DT) | ||
| + | |- | ||
| + | |6||BBa_I732020(RBS-lacZα-DT) | ||
| + | |- | ||
| + | |7||NC | ||
| + | |- | ||
| + | |8||100bp ladder | ||
| + | |} | ||
| + | [[File:igku_8162.jpg]]<br> | ||
</div> | </div> | ||
===Master Plate=== | ===Master Plate=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">Tatsui</span> |
{| class="wikitable" | {| class="wikitable" | ||
| - | !Number||Use LB plate (+ | + | !Number||Use LB plate (+CP) |
| + | |- | ||
| + | |1||BBa_I13504(RBS-GFP-DT)(1) | ||
| + | |- | ||
| + | |2||BBa_I13504(RBS-GFP-DT)(2) | ||
| + | |- | ||
| + | |3||BBa_K748002(lysis3) | ||
| + | |- | ||
| + | |4||BBa_R0061(Plux) | ||
| + | |- | ||
| + | |5||BBa_K117000(lysis1)(1) | ||
| + | |- | ||
| + | |6||BBa_K117000(lysis1)(2) | ||
| + | |- | ||
| + | |7||BBa_B0015(DT) | ||
|- | |- | ||
| - | | | + | |8||BBa_I732020(RBS-lacZα-DT) |
|} | |} | ||
| + | {| class="wikitable" | ||
| + | !Number||Use LB plate (+Amp) | ||
| + | |- | ||
| + | |1||BBa_I0462(RBS-luxR-DT)(1) | ||
| + | |- | ||
| + | |2||BBa_I0462(RBS-luxR-DT)(2) | ||
| + | |} | ||
</div> | </div> | ||
| + | |||
===Transformation=== | ===Transformation=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">Hirano</span> |
{| class="wikitable" | {| class="wikitable" | ||
!Name||Sample||Competent Cells||Total||Plate | !Name||Sample||Competent Cells||Total||Plate | ||
|- | |- | ||
| - | | | + | |pBR322||1µL||100µL(XL-10gold)||101µL||Amp |
|- | |- | ||
| - | | | + | |pBR322||1µL||50µL(takara)||51µL||Amp |
|- | |- | ||
| - | | || || || || | + | |Pbad/araC||2µL|||20µL||22µL||Kan |
| + | |- | ||
| + | |RBS-luxI-DT||2µL|||20µL||22µL||Kan | ||
|} | |} | ||
</div> | </div> | ||
| + | |||
===Liquid Culture=== | ===Liquid Culture=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">Yoshdia</span> |
{| class="wikitable" | {| class="wikitable" | ||
!Sample||medium | !Sample||medium | ||
|- | |- | ||
| - | |( | + | |8/15 BBa_I13504(RBS-GFP-DT))(1)||Plusgrow medium (+CP) |
| + | |- | ||
| + | |8/15 BBa_I13504(RBS-GFP-DT)(2)||Plusgrow medium (+CP) | ||
| + | |- | ||
| + | |8/15 BBa_I0462(RBS-luxR-DT)(2)||Plusgrow medium (+Amp) | ||
| + | |- | ||
| + | |8/15 BBa_K748002(lysis3)||Plusgrow medium (+CP) | ||
| + | |- | ||
| + | |8/15 BBa_R0061(Plux)||Plusgrow medium (+CP) | ||
| + | |- | ||
| + | |8/15 BBa_K117000(lysis1)(1)||Plusgrow medium (+CP) | ||
| + | |- | ||
| + | |8/15 BBa_K117000(lysis1)(2)||Plusgrow medium (+CP) | ||
| + | |- | ||
| + | |8/15 BBa_B0015(DT)||Plusgrow medium (+CP) | ||
| + | |- | ||
| + | |8/15 BBa_I732020(RBS-lacZα-DT)||Plusgrow medium (+CP) | ||
|- | |- | ||
| - | |||
|} | |} | ||
</div> | </div> | ||
Latest revision as of 19:31, 27 September 2013
Contents |
Aug 16
Colony PCR
| Sample | base pair |
|---|---|
| 8/15 BBa_I13504(RBS-GFP-DT)(1) | 1174 |
| 8/15 BBa_I13504(RBS-GFP-DT)(2) | 1174 |
| 8/15 BBa_I0462(RBS-luxR-DT)(1) | 1174 |
| 8/15 BBa_I0462(RBS-luxR-DT)(2) | 1174 |
| 8/15 BBa_K748002(lysis3) | 1053 |
| NC | -- |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 1min | 30cycles |
| Sample | base pair |
|---|---|
| 8/15 BBa_R0061(Plux) | 344 |
| 8/15 BBa_K117000(lysis1)(1) | 456 |
| 8/15 BBa_K117000(lysis1)(2) | 456 |
| 8/15 BBa_B0015(DT) | 443 |
| 8/15 BBa_I732020(RBS-lacZα-DT) | 703 |
| NC | -- |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 40s | 30cycles |
Electrophoresis
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | BBa_I13504(RBS-GFP-DT) -(1) |
| 3 | BBa_I13504(RBS-GFP-DT) -(2) |
| 4 | BBa_I0462(RBS-luxR-DT) -(1) |
| 5 | BBa_I0462(RBS-luxR-DT) -(2) |
| 6 | BBa_K748002(lysis3) |
| 7 | NC |
| 8 | 100bp ladder |
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | BBa_R0061(Plux) |
| 3 | BBa_K117000(lysis1) -(1) |
| 4 | BBa_K117000(lysis1) -(1) |
| 5 | BBa_B0015(DT) |
| 6 | BBa_I732020(RBS-lacZα-DT) |
| 7 | NC |
| 8 | 100bp ladder |
Master Plate
| Number | Use LB plate (+CP) |
|---|---|
| 1 | BBa_I13504(RBS-GFP-DT)(1) |
| 2 | BBa_I13504(RBS-GFP-DT)(2) |
| 3 | BBa_K748002(lysis3) |
| 4 | BBa_R0061(Plux) |
| 5 | BBa_K117000(lysis1)(1) |
| 6 | BBa_K117000(lysis1)(2) |
| 7 | BBa_B0015(DT) |
| 8 | BBa_I732020(RBS-lacZα-DT) |
| Number | Use LB plate (+Amp) |
|---|---|
| 1 | BBa_I0462(RBS-luxR-DT)(1) |
| 2 | BBa_I0462(RBS-luxR-DT)(2) |
Transformation
| Name | Sample | Competent Cells | Total | Plate |
|---|---|---|---|---|
| pBR322 | 1µL | 100µL(XL-10gold) | 101µL | Amp |
| pBR322 | 1µL | 50µL(takara) | 51µL | Amp |
| Pbad/araC | 2µL | 20µL | 22µL | Kan |
| RBS-luxI-DT | 2µL | 20µL | 22µL | Kan |
Liquid Culture
| Sample | medium |
|---|---|
| 8/15 BBa_I13504(RBS-GFP-DT))(1) | Plusgrow medium (+CP) |
| 8/15 BBa_I13504(RBS-GFP-DT)(2) | Plusgrow medium (+CP) |
| 8/15 BBa_I0462(RBS-luxR-DT)(2) | Plusgrow medium (+Amp) |
| 8/15 BBa_K748002(lysis3) | Plusgrow medium (+CP) |
| 8/15 BBa_R0061(Plux) | Plusgrow medium (+CP) |
| 8/15 BBa_K117000(lysis1)(1) | Plusgrow medium (+CP) |
| 8/15 BBa_K117000(lysis1)(2) | Plusgrow medium (+CP) |
| 8/15 BBa_B0015(DT) | Plusgrow medium (+CP) |
| 8/15 BBa_I732020(RBS-lacZα-DT) | Plusgrow medium (+CP) |
 "
"