Template:Kyoto/Notebook/Sep 9
From 2013.igem.org
(Difference between revisions)
(→Sep 9) |
(→Restriction Enzyme Digestion) |
||
| (74 intermediate revisions not shown) | |||
| Line 15: | Line 15: | ||
|2min||10s||30s||30s||30cycles | |2min||10s||30s||30s||30cycles | ||
|} | |} | ||
| - | |||
| - | |||
{| class="wikitable" | {| class="wikitable" | ||
!KaiB(plasmid)||10x buffer||dNTPs||MgSO4||Primer(661-RBS-KaiA-fwd-ver2)||Primer(kaiB-663-rev-ver2)||Kod-plus ver2||MilliQ||total | !KaiB(plasmid)||10x buffer||dNTPs||MgSO4||Primer(661-RBS-KaiA-fwd-ver2)||Primer(kaiB-663-rev-ver2)||Kod-plus ver2||MilliQ||total | ||
| Line 41: | Line 39: | ||
|2min||10s||30s||30s||30cycles | |2min||10s||30s||30s||30cycles | ||
|} | |} | ||
| + | {| class="wikitable" | ||
| + | !DT(plasmid)||10x buffer||dNTPs||MgSO4||Primer(664-DT-fwd-ver2)||Primer(DT-suffix-660-rev-ver2)||Kod-plus ver2||MilliQ||total | ||
| + | |- | ||
| + | |1.0||2.5||2.5||1.5||0.75||0.75||0.5||15.5||25 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94°C||98°C||58.5°C||68°C||-- | ||
| + | |- | ||
| + | |2min||10s||30s||30s||30cycles | ||
| + | |} | ||
| + | </div> | ||
| + | ===Gel Extraction=== | ||
| + | <!-- こっから --> | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Yoshida</span> | ||
| + | {| class="wikitable" | ||
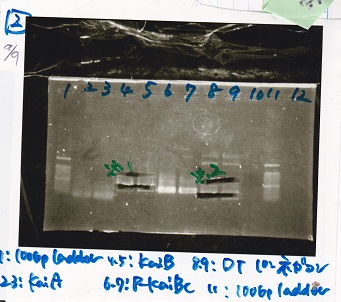
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||100bp ladder||-- | ||
| + | |- | ||
| + | |2||rowspan="2"|KaiA||rowspan="2"|-- | ||
| + | |- | ||
| + | |3 | ||
| + | |- | ||
| + | |4||rowspan="2"|KaiB||rowspan="2"|-- | ||
| + | |- | ||
| + | |5 | ||
| + | |- | ||
| + | |6||rowspan="2"|P-KaiBC||rowspan="2"|-- | ||
| + | |- | ||
| + | |7 | ||
| + | |- | ||
| + | |8||rowspan="2"|DT||rowspan="2"|-- | ||
| + | |- | ||
| + | |9 | ||
| + | |- | ||
| + | |10||NC||-- | ||
| + | |- | ||
| + | |11||100bp ladder||-- | ||
| + | |} | ||
| + | [[File:igku_992.jpg]]<br> | ||
| + | [[File:igku_993.jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Name||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |KaiC||28.5||1.26||11.92 | ||
| + | |- | ||
| + | |Plac||53.9||1.82||1.61 | ||
| + | |- | ||
| + | |KaiB||59.6||1.61||2.20 | ||
| + | |- | ||
| + | |KaiB||76.5||1.86||1.44 | ||
| + | |- | ||
| + | |DT||98.7||1.47||1.43 | ||
| + | |} | ||
| + | </div> | ||
| + | <!-- ここまでをコピーしてね --> | ||
| + | |||
| + | ===Miniprep=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {|class="wikitable" | ||
| + | !DNA||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |9/8 pSB1C3||83.2||1.45||0.76 | ||
| + | |- | ||
| + | |9/8 RBS-lysis2-DT||72.8||1.67||0.97 | ||
| + | |- | ||
| + | |9/8 spinach-DT||152.6||1.88||1.97 | ||
| + | |- | ||
| + | |Nuclease-Free water||-0.4||-14.30||0.25 | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Restriction Enzyme Digestion=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | ! ||9/9 pSB1C3||XbaI||PstI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||24µL||1µL||1µL||3µL||3µL||22µL||30µL* | ||
| + | |- | ||
| + | |NC||1.2µL||0µL||0µL||1µL||1µL||6.8 µL||10µL | ||
| + | |} | ||
| + | </div> | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | ! ||9/9 pSB1C3||XbaI||PstI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||27.4µL||1µL||1µL||3µL||3µL||22µL||30µL* | ||
| + | |- | ||
| + | |NC||1.3µL||0µL||0µL||1µL||1µL||6.7µL||10µL | ||
| + | |} | ||
| + | </div> | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | ! ||9/9 spinach-DT||XbaI||PstI||Buffer||BSA||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||10.8µL||1µL||1µL||3µL||3µL||22µL||30µL* | ||
| + | |- | ||
| + | |NC||0.5µL||0µL||0µL||1µL||1µL||7.5µL||10µL | ||
| + | |} | ||
| + | </div> | ||
| + | *"*"=be evaporated | ||
| + | |||
| + | ===Transformation=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Name||Sample||Competent Cells||Total||Plate | ||
| + | |- | ||
| + | |Pcon+RBS-luxI-DT||2µL||20µL||22µL||Amp | ||
| + | |- | ||
| + | |Pcon-RBS-luxR-DT+Plux-GFP||2µL||20µL||22µL|| | ||
| + | |- | ||
| + | |Ptet-PT181 antisense+spinach-DT-Pbad/araC+RBS-lux-DT||2µL||20µL||22µL|| | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Ligation=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No Name</span> | ||
| + | {| class="wikitable" | ||
| + | !state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | ||
| + | |- | ||
| + | |experiment||9/8 F1-attenuator(XbaI&PstI)||5.3µg||9/8 pSB1C3(XbaI&PstI)||8.9µg||3.5µg | ||
| + | |- | ||
| + | |experiment||9/8 F3m2-attenuator(XbaI&PstI)||4.5µg||9/8 pSB1C3(XbaI&PstI)||8.9µg||3.5µg | ||
| + | |- | ||
| + | |experiment||9/8 F1-antisense(XbaI&PstI)||1.7µg||9/8 pSB1C3(XbaI&PstI)||8.9µg||3.5µg | ||
| + | |- | ||
| + | |experiment||9/8 F6-antisense(XbaI&PstI)||2.9µg||9/8 pSB1C3(XbaI&PstI)||8.9µg||3.5µg | ||
| + | |- | ||
| + | |experiment||9/8 aptamer12-P(EcoRI&SpeI)||3.1µg||9/8 pSB1C3(EcoRI&SpeI)||8.8µg||3.5µg | ||
| + | |- | ||
| + | |experiment||9/8 aptamer12-1M(EcoRI&SpeI)||3.1µg||9/8 pSB1C(EcoRI&SpeI)3||8.8µg||3.5µg | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===M9 medium=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Shimizu & Hirano</span> | ||
| + | {| class="wikitable" | ||
| + | !EDTA consenlation||Na2HPO4||KH2PO4||NaCl||NH4Cl||Fe(Ⅲ)-EDTA||distilled water||total | ||
| + | |- | ||
| + | |1mMol||300mg||150mg||25mg||50mg||21mg||up to 10mL||10mL | ||
| + | |- | ||
| + | |10mMol||300mg||150mg||25mg||50mg||210.5mg||up to 10mL||10mL | ||
| + | |- | ||
| + | |50mMol||300mg||150mg||25mg||50mg||1052.7mg||up to 10mL||10mL | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Colony PCR=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||base pair | ||
| + | |- | ||
| + | |9/8 Plac-spinach-DT-(1)||659 | ||
| + | |- | ||
| + | |9/8 Plac-spinach-DT-(2)||659 | ||
| + | |- | ||
| + | |9/8 Pcon-pt181attenuator-DT-(1)||584 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94°C||94°C||55°C||68°C||-- | ||
| + | |- | ||
| + | |5min||30s||30s||40s||30cycles | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Gel Extraction=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
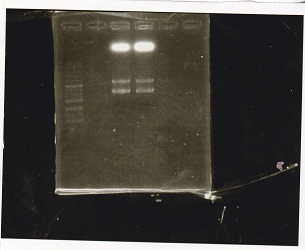
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||100bp ladder||-- | ||
| + | |-- | ||
| + | |2||rowspan="2"|Spinach-DT||rowspan="2"|XbaI+PstI | ||
| + | |- | ||
| + | |3 | ||
| + | |- | ||
| + | |5||rowspan="2"|RBS-lacZα-DT||rowspan="2"|XbaI+PstI | ||
| + | |- | ||
| + | |6 | ||
| + | |} | ||
| + | [[File:igku_995.jpg]]<br> | ||
| + | |||
| + | </div> | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||100bp ladder||-- | ||
| + | |-- | ||
| + | |3||rowspan="2"|pSB1C3||rowspan="2"|XbaI+PstI | ||
| + | |- | ||
| + | |4 | ||
| + | |} | ||
| + | [[File:igku_996.jpg]]<br> | ||
| + | |||
| + | </div> | ||
| + | |||
| + | ===Ligation=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | ||
| + | |- | ||
| + | |experiment||pSB1C3(XbaI+PstI)||xx µL||9/8 F1-attenuator(XbaI+PstI)||xx µL||xx µL | ||
| + | |- | ||
| + | |experiment||pSB1C3(XbaI+PstI)||xx µL||9/8 F3m2-attenuator(XbaI+PstI)||xx µL||xx µL | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===PCR=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Tatsui and Yoshida</span> | ||
| + | {| class="wikitable" | ||
| + | !KaiC||10x buffer KOD plus ver2||2M dNTP||MgSO4||Primer Mix(F&R)||Kod-plus ver2||MilliQ||total | ||
| + | |- | ||
| + | |0.5||2.5||2.5||1.5||1.5||0.5||16.0||25.0 | ||
| + | |- | ||
| + | |1.0||2.5||2.5||1.5||1.5||0.5||15.5||25.0 | ||
| + | |- | ||
| + | |2.0||2.5||2.5||1.5||1.5||0.5||14.5||25.0 | ||
| + | |- | ||
| + | |4.0||2.5||2.5||1.5||1.5||0.5||12.5||25.0 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94°C||98°C||57°C||68°C||-- | ||
| + | |- | ||
| + | |2min||10s||30s||2min||30cycles | ||
| + | |} | ||
| + | </div> | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | !Plac(100pg)||10x buffer KOD plus ver2||2M dNTP||MgSO4||Primer Mix(F&R)||Kod-plus ver2||MilliQ||total | ||
| + | |- | ||
| + | |0.3||2.5||2.5||1.5||1.5||0.5||16.2||25.0 | ||
| + | |- | ||
| + | |0.6||2.5||2.5||1.5||1.5||0.5||15.9||25.0 | ||
| + | |- | ||
| + | |1.2||2.5||2.5||1.5||1.5||0.5||15.3||25.0 | ||
| + | |- | ||
| + | |2.4||2.5||2.5||1.5||1.5||0.5||14.1||25.0 | ||
| + | |- | ||
| + | |0.0||2.5||2.5||1.5||1.5||0.5||16.5||25.0 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94°C||98°C||57°C||68°C||-- | ||
| + | |- | ||
| + | |2min||10s||30s||2min||30cycles | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Liquid Culture=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||medium | ||
| + | |- | ||
| + | |pSB2C3(master)-2||Plusgrow medium(+CP) | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Restriction Enzyme Digestion=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | ! ||8/17 lysis1-2||XbaI||PstI||BSA||Buffer||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||8.1µL||1µL||1µL||3µL||3µL||13.9µL||30µL | ||
| + | |- | ||
| + | |NC||0.4µL||0µL||0µL||1µL||1µL||7.6µL||10µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||8/17 lysis3-1||XbaI||PstI||BSA||Buffer||MilliQ||total | ||
| + | |- | ||
| + | |2 cuts||6.1µL||1µL||1µL||3µL||3µL||15.9µL||30µL | ||
| + | |- | ||
| + | |NC||0.3µL||0µL||0µL||1µL||1µL||7.7µL||10µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | ! ||9/15 Plac||XbaI||PstI||BSA||Buffer||MilliQ||total | ||
| + | |- | ||
| + | |1 cuts||12.1µL||0µL||1µL||3µL||3µL||10.9µL||30µL | ||
| + | |- | ||
| + | |NC||0.3µL||0µL||0µL||1µL||1µL||7.7µL||10µL | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===PCR=== | ||
| + | <!-- ここから --> | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Yoshida</span> | ||
| + | {| class="wikitable" | ||
| + | !KaiA(KaiABC) 100pg/µL||10x buffer KOD plus ver2||dNTPs||MgSO4||Primer Mix(Fwd&Rev)||KOD plus ver2||MilliQ||total | ||
| + | |- | ||
| + | |2.0||2.5||2.5||1.5||1.5||0.5||14.5||25 | ||
| + | |- | ||
| + | |4.0||2.5||2.5||1.5||1.5||0.5||12.5||25 | ||
| + | |- | ||
| + | |8.0||2.5||2.5||1.5||1.5||0.5||8.5||25 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94°C||98°C||60°C||68°C||-- | ||
| + | |- | ||
| + | |2min||10s||30s||1min||30cycles | ||
| + | |} | ||
| + | </div> | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | !PKaiBC 400pg/µL||10x buffer KOD plus ver2||dNTPs||MgSO4||Primer Mix(Fwd&Rev)||KOD plus ver2||MilliQ||total | ||
| + | |- | ||
| + | |0.5||2.5||2.5||1.5||1.5||0.5||16.0||25 | ||
| + | |- | ||
| + | |1.0||2.5||2.5||1.5||1.5||0.5||15.5||25 | ||
| + | |- | ||
| + | |2.0||2.5||2.5||1.5||1.5||0.5||14.5||25 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94°C||98°C||60°C||68°C||-- | ||
| + | |- | ||
| + | |2m||10s||30s||1m||30cycles | ||
| + | |} | ||
| + | </div> | ||
| + | ===Liquid Culture=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||medium | ||
| + | |- | ||
| + | |9/2 Spinach-DT||Plusgrow medium(+CP) | ||
| + | |- | ||
| + | |8/29 Plux-RBS-GFP-DT||Plusgrow medium | ||
| + | |- | ||
| + | |9/6 master plate ||Plusgrow medium(+Amp) | ||
| + | |- | ||
| + | |3 Pcon-PT181 attenuator||Plusgrow medium | ||
| + | |} | ||
| + | </div> | ||
| + | ===Gel Extraction=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
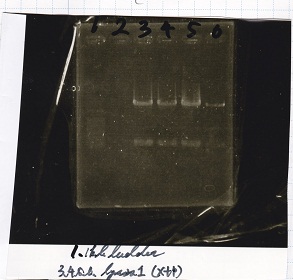
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||1kbp ladder||-- | ||
| + | |- | ||
| + | |3||rowspan="4"|lysis1||rowspan="4"|XbaI+PstI | ||
| + | |- | ||
| + | |4 | ||
| + | |- | ||
| + | |5 | ||
| + | |- | ||
| + | |6 | ||
| + | |} | ||
| + | [[File:igku_9910.jpg]]<br> | ||
| + | [[File:igku_9911.jpg]]<br> | ||
| + | </div> | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||1kbp ladder||-- | ||
| + | |- | ||
| + | |3||rowspan="4"|lysis3||rowspan="4"|XbaI+PstI | ||
| + | |- | ||
| + | |4 | ||
| + | |- | ||
| + | |5 | ||
| + | |- | ||
| + | |6 | ||
| + | |} | ||
| + | [[File:igku_9912.jpg]]<br> | ||
| + | [[File:igku_9913.jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Name||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |(´ω`)|| || || | ||
| + | |- | ||
| + | |(ΦωΦ^)|| || || | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Electrophoresis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Yoshida</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||1kb ladder||--||-- | ||
| + | |- | ||
| + | |2||Plac 100pg||--||-- | ||
| + | |- | ||
| + | |3||Plac 200pg||--||-- | ||
| + | |- | ||
| + | |4||Plac 400pg||--||-- | ||
| + | |- | ||
| + | |5||Plac 800pg||--||-- | ||
| + | |- | ||
| + | |6||NC||--||-- | ||
| + | |- | ||
| + | |7||1kbp ladder||--||-- | ||
| + | |} | ||
| + | [[File:igku_9914.jpg]]<br> | ||
</div> | </div> | ||
Latest revision as of 20:10, 27 September 2013
Contents |
Sep 9
PCR
| KaiA(plasmid) | 10x buffer | dNTPs | MgSO4 | Primer(661-RBS-KaiA-fwd) | Primer(kaiA-662-rev-ver2) | Kod-plus ver2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 1.0 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 15.5 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 58.5°C | 68°C | -- |
| 2min | 10s | 30s | 30s | 30cycles |
| KaiB(plasmid) | 10x buffer | dNTPs | MgSO4 | Primer(661-RBS-KaiA-fwd-ver2) | Primer(kaiB-663-rev-ver2) | Kod-plus ver2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 1.0 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 15.5 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 58.5°C | 68°C | -- |
| 2min | 10s | 30s | 30s | 30cycles |
| P-KaiBC(PCR products) | 10x buffer | dNTPs | MgSO4 | Primer(prefix-P-kaiBC-fwd-ver2) | Primer(P-KaiBC-suffix-rev-verw) | Kod-plus ver2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.7 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 15.8 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 58.5°C | 68°C | -- |
| 2min | 10s | 30s | 30s | 30cycles |
| DT(plasmid) | 10x buffer | dNTPs | MgSO4 | Primer(664-DT-fwd-ver2) | Primer(DT-suffix-660-rev-ver2) | Kod-plus ver2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 1.0 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 15.5 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 58.5°C | 68°C | -- |
| 2min | 10s | 30s | 30s | 30cycles |
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 2 | KaiA | -- |
| 3 | ||
| 4 | KaiB | -- |
| 5 | ||
| 6 | P-KaiBC | -- |
| 7 | ||
| 8 | DT | -- |
| 9 | ||
| 10 | NC | -- |
| 11 | 100bp ladder | -- |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| KaiC | 28.5 | 1.26 | 11.92 |
| Plac | 53.9 | 1.82 | 1.61 |
| KaiB | 59.6 | 1.61 | 2.20 |
| KaiB | 76.5 | 1.86 | 1.44 |
| DT | 98.7 | 1.47 | 1.43 |
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 9/8 pSB1C3 | 83.2 | 1.45 | 0.76 |
| 9/8 RBS-lysis2-DT | 72.8 | 1.67 | 0.97 |
| 9/8 spinach-DT | 152.6 | 1.88 | 1.97 |
| Nuclease-Free water | -0.4 | -14.30 | 0.25 |
Restriction Enzyme Digestion
| 9/9 pSB1C3 | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 24µL | 1µL | 1µL | 3µL | 3µL | 22µL | 30µL* |
| NC | 1.2µL | 0µL | 0µL | 1µL | 1µL | 6.8 µL | 10µL |
| 9/9 pSB1C3 | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 27.4µL | 1µL | 1µL | 3µL | 3µL | 22µL | 30µL* |
| NC | 1.3µL | 0µL | 0µL | 1µL | 1µL | 6.7µL | 10µL |
| 9/9 spinach-DT | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 10.8µL | 1µL | 1µL | 3µL | 3µL | 22µL | 30µL* |
| NC | 0.5µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL |
- "*"=be evaporated
Transformation
| Name | Sample | Competent Cells | Total | Plate |
|---|---|---|---|---|
| Pcon+RBS-luxI-DT | 2µL | 20µL | 22µL | Amp |
| Pcon-RBS-luxR-DT+Plux-GFP | 2µL | 20µL | 22µL | |
| Ptet-PT181 antisense+spinach-DT-Pbad/araC+RBS-lux-DT | 2µL | 20µL | 22µL |
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | 9/8 F1-attenuator(XbaI&PstI) | 5.3µg | 9/8 pSB1C3(XbaI&PstI) | 8.9µg | 3.5µg |
| experiment | 9/8 F3m2-attenuator(XbaI&PstI) | 4.5µg | 9/8 pSB1C3(XbaI&PstI) | 8.9µg | 3.5µg |
| experiment | 9/8 F1-antisense(XbaI&PstI) | 1.7µg | 9/8 pSB1C3(XbaI&PstI) | 8.9µg | 3.5µg |
| experiment | 9/8 F6-antisense(XbaI&PstI) | 2.9µg | 9/8 pSB1C3(XbaI&PstI) | 8.9µg | 3.5µg |
| experiment | 9/8 aptamer12-P(EcoRI&SpeI) | 3.1µg | 9/8 pSB1C3(EcoRI&SpeI) | 8.8µg | 3.5µg |
| experiment | 9/8 aptamer12-1M(EcoRI&SpeI) | 3.1µg | 9/8 pSB1C(EcoRI&SpeI)3 | 8.8µg | 3.5µg |
M9 medium
| EDTA consenlation | Na2HPO4 | KH2PO4 | NaCl | NH4Cl | Fe(Ⅲ)-EDTA | distilled water | total |
|---|---|---|---|---|---|---|---|
| 1mMol | 300mg | 150mg | 25mg | 50mg | 21mg | up to 10mL | 10mL |
| 10mMol | 300mg | 150mg | 25mg | 50mg | 210.5mg | up to 10mL | 10mL |
| 50mMol | 300mg | 150mg | 25mg | 50mg | 1052.7mg | up to 10mL | 10mL |
Colony PCR
| Sample | base pair |
|---|---|
| 9/8 Plac-spinach-DT-(1) | 659 |
| 9/8 Plac-spinach-DT-(2) | 659 |
| 9/8 Pcon-pt181attenuator-DT-(1) | 584 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 40s | 30cycles |
Gel Extraction
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | pSB1C3(XbaI+PstI) | xx µL | 9/8 F1-attenuator(XbaI+PstI) | xx µL | xx µL |
| experiment | pSB1C3(XbaI+PstI) | xx µL | 9/8 F3m2-attenuator(XbaI+PstI) | xx µL | xx µL |
PCR
| KaiC | 10x buffer KOD plus ver2 | 2M dNTP | MgSO4 | Primer Mix(F&R) | Kod-plus ver2 | MilliQ | total |
|---|---|---|---|---|---|---|---|
| 0.5 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 16.0 | 25.0 |
| 1.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 15.5 | 25.0 |
| 2.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 14.5 | 25.0 |
| 4.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 12.5 | 25.0 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 57°C | 68°C | -- |
| 2min | 10s | 30s | 2min | 30cycles |
| Plac(100pg) | 10x buffer KOD plus ver2 | 2M dNTP | MgSO4 | Primer Mix(F&R) | Kod-plus ver2 | MilliQ | total |
|---|---|---|---|---|---|---|---|
| 0.3 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 16.2 | 25.0 |
| 0.6 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 15.9 | 25.0 |
| 1.2 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 15.3 | 25.0 |
| 2.4 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 14.1 | 25.0 |
| 0.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 16.5 | 25.0 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 57°C | 68°C | -- |
| 2min | 10s | 30s | 2min | 30cycles |
Liquid Culture
| Sample | medium |
|---|---|
| pSB2C3(master)-2 | Plusgrow medium(+CP) |
Restriction Enzyme Digestion
| 8/17 lysis1-2 | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 8.1µL | 1µL | 1µL | 3µL | 3µL | 13.9µL | 30µL |
| NC | 0.4µL | 0µL | 0µL | 1µL | 1µL | 7.6µL | 10µL |
| 8/17 lysis3-1 | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 6.1µL | 1µL | 1µL | 3µL | 3µL | 15.9µL | 30µL |
| NC | 0.3µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL |
| 9/15 Plac | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 1 cuts | 12.1µL | 0µL | 1µL | 3µL | 3µL | 10.9µL | 30µL |
| NC | 0.3µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL |
PCR
| KaiA(KaiABC) 100pg/µL | 10x buffer KOD plus ver2 | dNTPs | MgSO4 | Primer Mix(Fwd&Rev) | KOD plus ver2 | MilliQ | total |
|---|---|---|---|---|---|---|---|
| 2.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 14.5 | 25 |
| 4.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 12.5 | 25 |
| 8.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 8.5 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 60°C | 68°C | -- |
| 2min | 10s | 30s | 1min | 30cycles |
| PKaiBC 400pg/µL | 10x buffer KOD plus ver2 | dNTPs | MgSO4 | Primer Mix(Fwd&Rev) | KOD plus ver2 | MilliQ | total |
|---|---|---|---|---|---|---|---|
| 0.5 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 16.0 | 25 |
| 1.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 15.5 | 25 |
| 2.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 14.5 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 60°C | 68°C | -- |
| 2m | 10s | 30s | 1m | 30cycles |
Liquid Culture
| Sample | medium |
|---|---|
| 9/2 Spinach-DT | Plusgrow medium(+CP) |
| 8/29 Plux-RBS-GFP-DT | Plusgrow medium |
| 9/6 master plate | Plusgrow medium(+Amp) |
| 3 Pcon-PT181 attenuator | Plusgrow medium |
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | -- |
| 3 | lysis3 | XbaI+PstI |
| 4 | ||
| 5 | ||
| 6 |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| (´ω`) | |||
| (ΦωΦ^) |
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 1kb ladder | -- | -- |
| 2 | Plac 100pg | -- | -- |
| 3 | Plac 200pg | -- | -- |
| 4 | Plac 400pg | -- | -- |
| 5 | Plac 800pg | -- | -- |
| 6 | NC | -- | -- |
| 7 | 1kbp ladder | -- | -- |
 "
"