Template:Kyoto/Notebook/Sep 17
From 2013.igem.org
(Difference between revisions)
(→PCR) |
(→Gel Extraction) |
||
| (34 intermediate revisions not shown) | |||
| Line 6: | Line 6: | ||
!Sample||medium | !Sample||medium | ||
|- | |- | ||
| - | | | + | |Spinach-DT||LB medium(+CP) |
|- | |- | ||
| - | |Pcon- | + | |Pcon-Spinach-DT||LB medium(+Amp) |
| + | |- | ||
| + | |Pcon-pT181 antisense-Spinach-DT||LB medium(+Amp) | ||
|} | |} | ||
</div> | </div> | ||
| + | |||
===PCR=== | ===PCR=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">Hirano</span> | <span class="author">Hirano</span> | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !Pcon- | + | !Pcon-tetR aptamer-DT(VF)||BigDye||5x buffer||primer(VF)||MilliQ||total |
| + | |- | ||
| + | |1.3||0.5||1.75||0.5||0.45||10.5 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !Pcon-tetR aptamer-DT(VR)||BigDye||5x buffer||primer(VR)||MilliQ||total | ||
|- | |- | ||
|1.3||0.5||1.75||0.5||0.45||10.5 | |1.3||0.5||1.75||0.5||0.45||10.5 | ||
| Line 23: | Line 30: | ||
!PreDenature||Denature||Annealing||Extension||cycle | !PreDenature||Denature||Annealing||Extension||cycle | ||
|- | |- | ||
| - | |96°C||96°C||50°C||60°C||-- | + | |96 °C||96 °C||50 °C||60 °C||-- |
|- | |- | ||
| - | | | + | |2 min||10 sec||5 sec||4 min||30 cycles |
|} | |} | ||
</div> | </div> | ||
| - | + | ||
| + | ===Electrophoresis=== | ||
<div class="experiment"> | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! | + | !Lane||Sample||Enzyme1||Enzyme2 |
|- | |- | ||
| - | |1 | + | |1||1kbp ladder||--||-- |
| - | + | ||
| - | + | ||
| - | + | ||
|- | |- | ||
| - | | | + | |2||Pcon-RBS-tetR-DT||XbaI||PstI |
|- | |- | ||
| - | | | + | |3||Pcon-RBS-tetR-DT||--||-- |
| + | |- | ||
| + | |4||pSB4K5||XbaI||PstI | ||
| + | |- | ||
| + | |5||pSB4K5||--||-- | ||
| + | |- | ||
| + | |6||pSB1C3||EcoRI||SpeI | ||
| + | |- | ||
| + | |7||pSB1C3||EcoRI||PstI | ||
| + | |- | ||
| + | |8||pSB1C3||--||-- | ||
| + | |- | ||
| + | |9||pT181 attenuator||EcoRI||SpeI | ||
| + | |- | ||
| + | |10||pT181 attenuator||--||-- | ||
| + | |- | ||
| + | |11||1kbp ladder||--||-- | ||
|} | |} | ||
| + | [[File:igku_9171.jpg]]<br> | ||
</div> | </div> | ||
| - | + | ||
===Ligation=== | ===Ligation=== | ||
| - | |||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">No Name</span> |
{| class="wikitable" | {| class="wikitable" | ||
!state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | !state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | ||
|- | |- | ||
| - | |experiment|| | + | |experiment||8/28 Plux (SpeI & PstI)||5.0 µL||9/13 RBS-lysis1-DT (XbaI & PstI)||3.5 µL||3.5 µL |
| + | |- | ||
| + | |experiment||8/28 Plux (SpeI & PstI)||5.0 µL||9/13 RBS-lysis2-DT (XbaI & PstI)||3.6 µL||3.5 µL | ||
| + | |- | ||
| + | |experiment||8/28 Plux (SpeI & PstI)||11.0 µL||9/13 RBS-lysis3-DT (XbaI & PstI)||9.5 µL||3.5 µL | ||
| + | |- | ||
| + | |experiment||9/14 Plac (SpeI & PstI)||2.4 µL||9/16 pT181 attenuator-DT (XbaI & PstI)||4.8 µL||3.5 µL | ||
| + | |- | ||
| + | |experiment||9/14 Plac (SpeI & PstI)||2.4 µL||9/3 pT181 antisense (XbaI & PstI)||2.0 µL||2.2 µL | ||
| + | |- | ||
| + | |experiment||9/14 Plac (SpeI & PstI)||2.4 µL||9/10 aptamer 12_1R-DT (XbaI & PstI)||1.8 µL||2.1 µL | ||
| + | |- | ||
| + | |experiment||9/13 pSB1C3 (XbaI & PstI)||2.0 µL||9/15 Pcon-PT181 antisense-Spinach-DT (XbaI & PstI)||12.0 µL||3.5 µL | ||
| + | |- | ||
| + | |experiment||9/16 pSB1C3 (EcoRI & SpeI)||1.0 µL||9/15 Pcon-Spinach-DT (EcoRI & SpeI)||4.1 µL||2.5 µL | ||
| + | |- | ||
| + | |experiment||9/16 pSB1C3 (EcoRI & SpeI)||1.0 µL||9/15 Pcon-RBS-tetR-DT (EcoRI & SpeI)||2.0 µL||1.5 µL | ||
| + | |- | ||
| + | |experiment||9/16 pSB1C3 (EcoRI & SpeI)||1.0 µL||9/15 Pcon-PT181 attenuator-DT (EcoRI & SpeI)||2.4 µL||1.7 µL | ||
| + | |- | ||
| + | |experiment||9/16 pSB1C3 (EcoRI & SpeI)||1.0 µL||9/15 Pcon-aptamer 12_1R-DT (EcoRI & SpeI)||2.0 µL||1.5 µL | ||
| + | |- | ||
| + | |experiment||9/13 pSB1C3 (XbaI & PstI)||2.0 µL||9/16 Pcon-pT181-attenuator (XbaI & PstI)||4.4 µL||3.2 µL | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Gel Extraction=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No Name</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||1kbp ladder||-- | ||
| + | |- | ||
| + | |2||rowspan="3"|pT181 attenuator -(2)||rowspan="3"|EcoRI & SpeI | ||
| + | |- | ||
| + | |3 | ||
| + | |- | ||
| + | |4 | ||
| + | |} | ||
| + | [[File:igku_9172.jpg]]<br> | ||
| + | [[File:igku_9173.jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Name||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |pT181 attenuator (EcoI & SpeI)||9.2||1.15||0.12 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||1kbp ladder||-- | ||
| + | |- | ||
| + | |2||rowspan="3"|pSB1C3||rowspan="3"|EcoRI & SpeI | ||
| + | |- | ||
| + | |3 | ||
| + | |- | ||
| + | |4 | ||
| + | |} | ||
| + | [[File:igku_9174.jpg]]<br> | ||
| + | [[File:igku_9175.jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Name||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |pSB1C3||10.0||1.84||0.40 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||1kbp ladder||-- | ||
| + | |- | ||
| + | |2||rowspan="3"|pSB4K5||rowspan="3"|XbaI & PstI | ||
| + | |- | ||
| + | |3 | ||
| + | |- | ||
| + | |4 | ||
| + | |} | ||
| + | [[File:igku_9176.jpg]]<br> | ||
| + | [[File:igku_9177.jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Name||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |pSB4K5||7.6||1.81||0.32 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||1kbp ladder||-- | ||
| + | |- | ||
| + | |2||rowspan="3"|Pcon-RBS-tetR-DT||rowspan="3"|XbaI & PstI | ||
| + | |- | ||
| + | |3 | ||
| + | |- | ||
| + | |4 | ||
| + | |} | ||
| + | [[File:igku_9178.jpg]]<br> | ||
| + | [[File:igku_9179.jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Name||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |Pcon-RBS-tetR-DT||8.0||2.40||1.14 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1||1kbp ladder||-- | ||
| + | |- | ||
| + | |2||rowspan="3"|pSB1C3||rowspan="3"|EcoRI & PstI | ||
| + | |- | ||
| + | |3 | ||
| + | |- | ||
| + | |4 | ||
| + | |} | ||
| + | [[File:igku_91710.jpg]]<br> | ||
| + | [[File:igku_91711.jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Name||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |pSB1C3||9.1||2.17||0.35 | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Mutation PCR=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Kinose</span> | ||
| + | {| class="wikitable" | ||
| + | !pC KaiABC(100pg/µL)||Prime STAR(2x)||Primer(KaiA-BsaI MuT-Fwd)||Primer(KaiA-BsaI MuT-Rev)||MilliQ||total | ||
| + | |- | ||
| + | |1.0||25.0||1.0||1.0||22.0||50.0 | ||
| + | |- | ||
| + | |1.5||25.0||1.0||1.0||21.5||50.0 | ||
| + | |- | ||
| + | |2.0||25.0||1.0||1.0||21.0||50.0 | ||
| + | |- | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |96 °C||98 °C||65 °C||72 °C||-- | ||
|- | |- | ||
| - | | | + | |5 min||10 s||15 s||40 s||30 cycles |
|} | |} | ||
</div> | </div> | ||
| - | |||
Latest revision as of 05:57, 26 September 2013
Contents |
Sep 17
Liquid Culture
| Sample | medium |
|---|---|
| Spinach-DT | LB medium(+CP) |
| Pcon-Spinach-DT | LB medium(+Amp) |
| Pcon-pT181 antisense-Spinach-DT | LB medium(+Amp) |
PCR
| Pcon-tetR aptamer-DT(VF) | BigDye | 5x buffer | primer(VF) | MilliQ | total |
|---|---|---|---|---|---|
| 1.3 | 0.5 | 1.75 | 0.5 | 0.45 | 10.5 |
| Pcon-tetR aptamer-DT(VR) | BigDye | 5x buffer | primer(VR) | MilliQ | total |
|---|---|---|---|---|---|
| 1.3 | 0.5 | 1.75 | 0.5 | 0.45 | 10.5 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 96 °C | 96 °C | 50 °C | 60 °C | -- |
| 2 min | 10 sec | 5 sec | 4 min | 30 cycles |
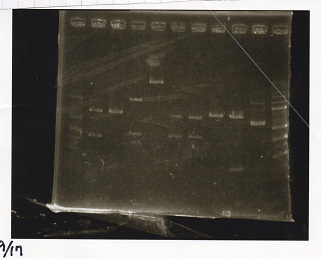
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 1kbp ladder | -- | -- |
| 2 | Pcon-RBS-tetR-DT | XbaI | PstI |
| 3 | Pcon-RBS-tetR-DT | -- | -- |
| 4 | pSB4K5 | XbaI | PstI |
| 5 | pSB4K5 | -- | -- |
| 6 | pSB1C3 | EcoRI | SpeI |
| 7 | pSB1C3 | EcoRI | PstI |
| 8 | pSB1C3 | -- | -- |
| 9 | pT181 attenuator | EcoRI | SpeI |
| 10 | pT181 attenuator | -- | -- |
| 11 | 1kbp ladder | -- | -- |
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | 8/28 Plux (SpeI & PstI) | 5.0 µL | 9/13 RBS-lysis1-DT (XbaI & PstI) | 3.5 µL | 3.5 µL |
| experiment | 8/28 Plux (SpeI & PstI) | 5.0 µL | 9/13 RBS-lysis2-DT (XbaI & PstI) | 3.6 µL | 3.5 µL |
| experiment | 8/28 Plux (SpeI & PstI) | 11.0 µL | 9/13 RBS-lysis3-DT (XbaI & PstI) | 9.5 µL | 3.5 µL |
| experiment | 9/14 Plac (SpeI & PstI) | 2.4 µL | 9/16 pT181 attenuator-DT (XbaI & PstI) | 4.8 µL | 3.5 µL |
| experiment | 9/14 Plac (SpeI & PstI) | 2.4 µL | 9/3 pT181 antisense (XbaI & PstI) | 2.0 µL | 2.2 µL |
| experiment | 9/14 Plac (SpeI & PstI) | 2.4 µL | 9/10 aptamer 12_1R-DT (XbaI & PstI) | 1.8 µL | 2.1 µL |
| experiment | 9/13 pSB1C3 (XbaI & PstI) | 2.0 µL | 9/15 Pcon-PT181 antisense-Spinach-DT (XbaI & PstI) | 12.0 µL | 3.5 µL |
| experiment | 9/16 pSB1C3 (EcoRI & SpeI) | 1.0 µL | 9/15 Pcon-Spinach-DT (EcoRI & SpeI) | 4.1 µL | 2.5 µL |
| experiment | 9/16 pSB1C3 (EcoRI & SpeI) | 1.0 µL | 9/15 Pcon-RBS-tetR-DT (EcoRI & SpeI) | 2.0 µL | 1.5 µL |
| experiment | 9/16 pSB1C3 (EcoRI & SpeI) | 1.0 µL | 9/15 Pcon-PT181 attenuator-DT (EcoRI & SpeI) | 2.4 µL | 1.7 µL |
| experiment | 9/16 pSB1C3 (EcoRI & SpeI) | 1.0 µL | 9/15 Pcon-aptamer 12_1R-DT (EcoRI & SpeI) | 2.0 µL | 1.5 µL |
| experiment | 9/13 pSB1C3 (XbaI & PstI) | 2.0 µL | 9/16 Pcon-pT181-attenuator (XbaI & PstI) | 4.4 µL | 3.2 µL |
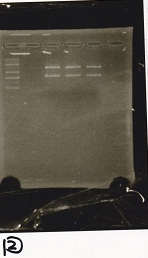
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | -- |
| 2 | pT181 attenuator -(2) | EcoRI & SpeI |
| 3 | ||
| 4 |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| pT181 attenuator (EcoI & SpeI) | 9.2 | 1.15 | 0.12 |
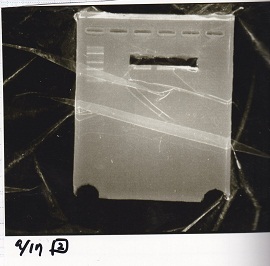
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | -- |
| 2 | pSB1C3 | EcoRI & SpeI |
| 3 | ||
| 4 |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| pSB1C3 | 10.0 | 1.84 | 0.40 |
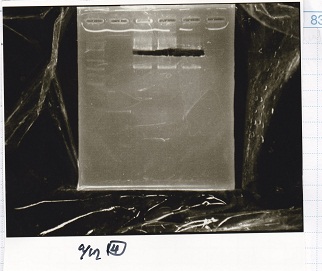
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | -- |
| 2 | pSB4K5 | XbaI & PstI |
| 3 | ||
| 4 |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| pSB4K5 | 7.6 | 1.81 | 0.32 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | -- |
| 2 | Pcon-RBS-tetR-DT | XbaI & PstI |
| 3 | ||
| 4 |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Pcon-RBS-tetR-DT | 8.0 | 2.40 | 1.14 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | -- |
| 2 | pSB1C3 | EcoRI & PstI |
| 3 | ||
| 4 |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| pSB1C3 | 9.1 | 2.17 | 0.35 |
Mutation PCR
| pC KaiABC(100pg/µL) | Prime STAR(2x) | Primer(KaiA-BsaI MuT-Fwd) | Primer(KaiA-BsaI MuT-Rev) | MilliQ | total |
|---|---|---|---|---|---|
| 1.0 | 25.0 | 1.0 | 1.0 | 22.0 | 50.0 |
| 1.5 | 25.0 | 1.0 | 1.0 | 21.5 | 50.0 |
| 2.0 | 25.0 | 1.0 | 1.0 | 21.0 | 50.0 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 96 °C | 98 °C | 65 °C | 72 °C | -- |
| 5 min | 10 s | 15 s | 40 s | 30 cycles |
 "
"