Team:Buenos Aires/ res rgfp
From 2013.igem.org
(→Quantitative measure of HSL production by pArs LuxI construction (Bba_K1106008)) |
FedeVignale (Talk | contribs) (→AHL production tested with Chromobacterium violaceum) |
||
| (15 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
<div id="external"> | <div id="external"> | ||
| - | = | + | =LuxI under Arsenite Inducible Promoter ([http://parts.igem.org/Part:Bba_K1106008 Bba_K1106008]) characterization= |
| - | Chromobacterium violaceum | + | == '''AHL production tested with Chromobacterium violaceum''' == |
| - | Materials | + | '''Materials''' |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | *Top Agar petri dishes with two layers (agar 0,5% and regular agar 1.5%) | |
| + | *Chromobacterium violaceum (CV026 strain) | ||
| + | *TY medium | ||
| + | *LB medium | ||
| + | *Rhizobium leguminosarum as a positive control | ||
| + | *E. coli DH5α harbouring our construct pArs_LuxI | ||
| - | + | '''Method''' | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | In order to asess if LuxI generator under arsenic sensitive promoter synthesizes AHL (a quorum sensing molecule) when is induced with arsenite, a top agar petri dish was prepared. A layer of sterile LB-agar 1,5% was poured and on top of it an additional layer of LB-agar 0,5% mixed with Chromobacterium v., a bacteria that in presence of certain AHLs turns purple due to the production of violacein. Wells of a volume around 50 ul were performed on the top layer to inoculate different cultures into them. | |
| - | + | Besides, four different cultures were grown overnight: | |
| + | |||
| + | · A Rhizobium leguminosarum culture, grown at 30 ºC in TY medium, as a positive control. | ||
| - | · | + | · Two ''E. coli'' cultures (DH5alpha) harbouring the LuxI enzyme gene of Vibrio fischeri, under the control of an arsenite inducible promoter. One of them was added with 1000 ppb of arsenite. |
| - | + | ||
| + | · An ''E. coli'' culture without the codifying sequence of LuxI, as a negative control. | ||
| - | + | Finally, the petri dish, with Chromobacterium v. on the top layer, was inoculated into the wells with samples of 50 ul of each culture and grown overnight at 30ºC. As Chromobacterium v. is a lactone sensitive bacteria, this method allows to detect AHL presence in an easy and very intuitive way by observing the appearance of a violet halo around the inoculation wells which means a positive result. | |
| - | ''' | + | '''Results''' |
| - | + | [[File:LuxIfede.jpg|center|800px]] | |
| - | + | · Inoculated wells with Rhizobium leguminosarum, the positive control, and the ''E.coli'' carrying LuxI sequence that was previously induced with 1000 ppb of arsenite, shown positive results. | |
| - | + | · The negative control without LuxI coding sequence and the ''E.coli'' carrying LuxI sequence without the addition of arsenite, shown negative results. | |
| - | + | == '''Future experiment: quantitative measure of AHL production by pArs LuxI construction''' == | |
| - | + | '''Method''' | |
| - | + | Four different cultures will be grown overnight: | |
| - | + | • An E. coli culture carrying two plasmids that encode an incoherent feedforward system,designed by Basu et al. ([http://www.ncbi.nlm.nih.gov/pubmed/?term=basu+2004+gfp Basu et al 2004]), which is inducible by Vibrio fischeri’s Lux system. This plasmids were gently provided by Ron Weiss’ lab. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | plasmids | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | • A Rhizobium leguminosarum culture, grown at 30 ºC in TY medium, as a positive control. | ||
| - | + | • Two E. coli cultures (DH5alpha) harbouring the LuxI enzyme gene of Vibrio fischeri, under the control of an arsenite inducible promoter (pArs). One of them was added with 1000 ppb of arsenite. | |
| - | + | • An E. coli culture (DH5alpha) that doesn´t have the LuxI gene. | |
| - | + | [[File:Basu_diseño.jpg|400px|]] | |
| - | + | The following day, E. coli culture carrying the incoherent feedforward plasmids will be split in four tubes and centrifuged. We will keep the cells, and discard the supernatant. Besides, the other four cultures will centrifuged. We will keep the supernatant and discard the cells. This four conditioned media will be used to resuspend the cells carrying Basu’s incoherent feedforward plasmids. | |
| - | + | Cells will be incubated at 37 ºC and every 15 minutes we will take an aliquot of each culture, during 105 minutes after the beginning of the induction. | |
| - | + | Afterwards, we will measure GFP production at each condition over the time. | |
</div> | </div> | ||
Latest revision as of 02:34, 28 September 2013
LuxI under Arsenite Inducible Promoter ([http://parts.igem.org/Part:Bba_K1106008 Bba_K1106008]) characterization
AHL production tested with Chromobacterium violaceum
Materials
- Top Agar petri dishes with two layers (agar 0,5% and regular agar 1.5%)
- Chromobacterium violaceum (CV026 strain)
- TY medium
- LB medium
- Rhizobium leguminosarum as a positive control
- E. coli DH5α harbouring our construct pArs_LuxI
Method
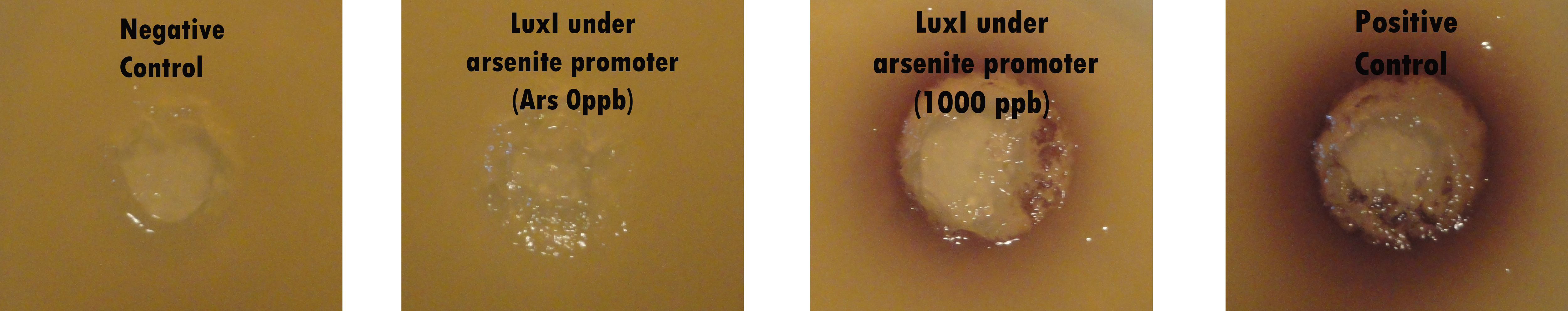
In order to asess if LuxI generator under arsenic sensitive promoter synthesizes AHL (a quorum sensing molecule) when is induced with arsenite, a top agar petri dish was prepared. A layer of sterile LB-agar 1,5% was poured and on top of it an additional layer of LB-agar 0,5% mixed with Chromobacterium v., a bacteria that in presence of certain AHLs turns purple due to the production of violacein. Wells of a volume around 50 ul were performed on the top layer to inoculate different cultures into them.
Besides, four different cultures were grown overnight:
· A Rhizobium leguminosarum culture, grown at 30 ºC in TY medium, as a positive control.
· Two E. coli cultures (DH5alpha) harbouring the LuxI enzyme gene of Vibrio fischeri, under the control of an arsenite inducible promoter. One of them was added with 1000 ppb of arsenite.
· An E. coli culture without the codifying sequence of LuxI, as a negative control.
Finally, the petri dish, with Chromobacterium v. on the top layer, was inoculated into the wells with samples of 50 ul of each culture and grown overnight at 30ºC. As Chromobacterium v. is a lactone sensitive bacteria, this method allows to detect AHL presence in an easy and very intuitive way by observing the appearance of a violet halo around the inoculation wells which means a positive result.
Results
· Inoculated wells with Rhizobium leguminosarum, the positive control, and the E.coli carrying LuxI sequence that was previously induced with 1000 ppb of arsenite, shown positive results.
· The negative control without LuxI coding sequence and the E.coli carrying LuxI sequence without the addition of arsenite, shown negative results.
Future experiment: quantitative measure of AHL production by pArs LuxI construction
Method
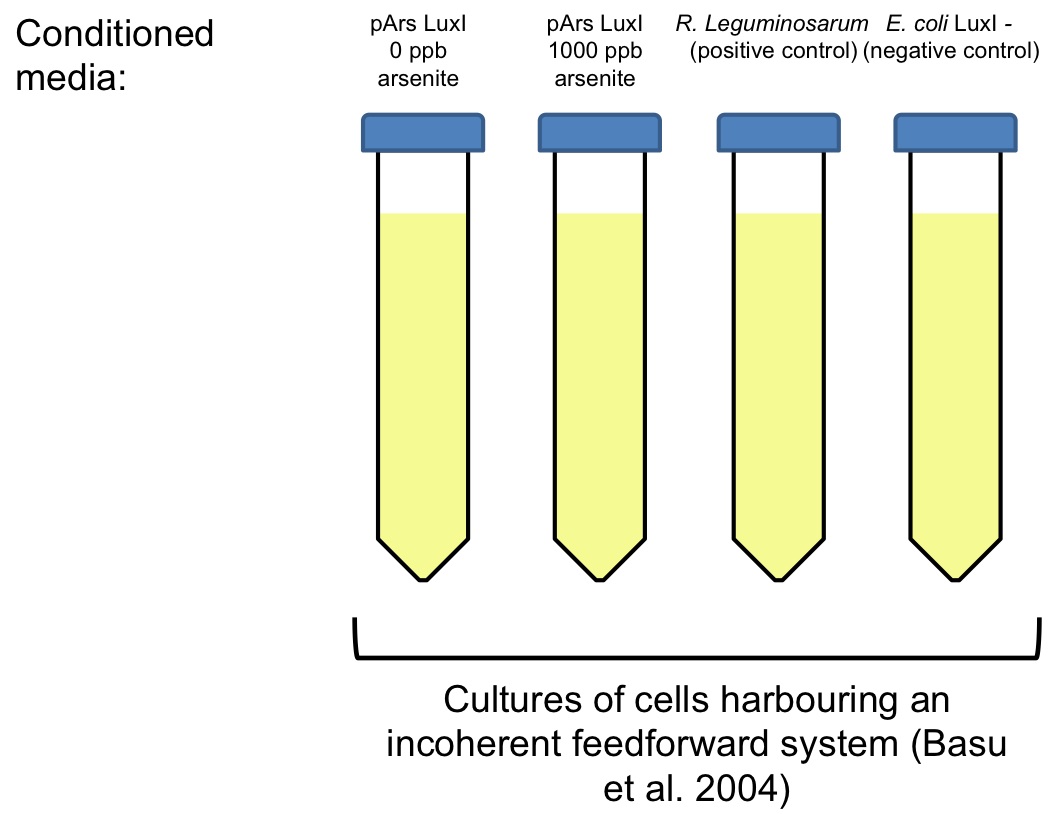
Four different cultures will be grown overnight:
• An E. coli culture carrying two plasmids that encode an incoherent feedforward system,designed by Basu et al. ([http://www.ncbi.nlm.nih.gov/pubmed/?term=basu+2004+gfp Basu et al 2004]), which is inducible by Vibrio fischeri’s Lux system. This plasmids were gently provided by Ron Weiss’ lab.
• A Rhizobium leguminosarum culture, grown at 30 ºC in TY medium, as a positive control.
• Two E. coli cultures (DH5alpha) harbouring the LuxI enzyme gene of Vibrio fischeri, under the control of an arsenite inducible promoter (pArs). One of them was added with 1000 ppb of arsenite.
• An E. coli culture (DH5alpha) that doesn´t have the LuxI gene.
The following day, E. coli culture carrying the incoherent feedforward plasmids will be split in four tubes and centrifuged. We will keep the cells, and discard the supernatant. Besides, the other four cultures will centrifuged. We will keep the supernatant and discard the cells. This four conditioned media will be used to resuspend the cells carrying Basu’s incoherent feedforward plasmids.
Cells will be incubated at 37 ºC and every 15 minutes we will take an aliquot of each culture, during 105 minutes after the beginning of the induction.
Afterwards, we will measure GFP production at each condition over the time.
 "
"