Template:Kyoto/Notebook/Sep 4
From 2013.igem.org
(Difference between revisions)
(→Electrophoresis) |
(→Electrophoresis) |
||
| (33 intermediate revisions not shown) | |||
| Line 12: | Line 12: | ||
|experiment||9/3 DT(EcoRI&XbaI) ||3.0µL ||9/3 RBS-lysis3||8.8µL ||4.0µL | |experiment||9/3 DT(EcoRI&XbaI) ||3.0µL ||9/3 RBS-lysis3||8.8µL ||4.0µL | ||
|- | |- | ||
| - | |experiment||9/3 DT(EcoRI&XbaI) ||3.0µL ||9/3 | + | |experiment||9/3 DT(EcoRI&XbaI) ||3.0µL ||9/3 aptamer12_1R(EcoRI&SpeI) ||1.9µL ||2.45µL |
|- | |- | ||
|experiment||9/2 pSB1C3(XbaI&PstI)||10.6µL ||9/3 pT181attenuator (XbaI& PstI)||3.2µL ||4.0µL | |experiment||9/2 pSB1C3(XbaI&PstI)||10.6µL ||9/3 pT181attenuator (XbaI& PstI)||3.2µL ||4.0µL | ||
| Line 18: | Line 18: | ||
|experiment||9/2 pSB1C3(XbaI&PstI)||9.6µL ||9/3 pT181antisense (XbaI& PstI)||2.1µL ||4.0µL | |experiment||9/2 pSB1C3(XbaI&PstI)||9.6µL ||9/3 pT181antisense (XbaI& PstI)||2.1µL ||4.0µL | ||
|- | |- | ||
| - | |experiment||9/2 pSB1C3(EcoRI&SpeI)||16.7µL ||9/3 | + | |experiment||9/2 pSB1C3(EcoRI&SpeI)||16.7µL ||9/3 aptamer12_1R(EcoRI&SpeI) ||2.0µL ||4.0µL |
|} | |} | ||
incubate 16 °C 1 hour | incubate 16 °C 1 hour | ||
</div> | </div> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
===Transformation=== | ===Transformation=== | ||
<div class="experiment"> | <div class="experiment"> | ||
| Line 41: | Line 34: | ||
|9/4 RBS-lysis3-DT ||2µL||20µL||Amp | |9/4 RBS-lysis3-DT ||2µL||20µL||Amp | ||
|- | |- | ||
| - | |9/4 | + | |9/4 aptamer12_1R-DT||2µL||20µL||Amp |
|- | |- | ||
|9/4 pT181attenuator –pSB1C3||2µL||20µL||Amp | |9/4 pT181attenuator –pSB1C3||2µL||20µL||Amp | ||
| Line 47: | Line 40: | ||
|9/4 pT181antisense-pSB1C3 ||2µL||20µL||Amp | |9/4 pT181antisense-pSB1C3 ||2µL||20µL||Amp | ||
|- | |- | ||
| - | |9/4 | + | |9/4 aptamer12_1R –pSB1C3||2µL||20µL||Amp |
|- | |- | ||
|9/4 pSB4K5(2013plate5 56) ||2µL||20µL||Amp | |9/4 pSB4K5(2013plate5 56) ||2µL||20µL||Amp | ||
| Line 83: | Line 76: | ||
|3||8/10 Plac(CP)-2 | |3||8/10 Plac(CP)-2 | ||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{| class="wikitable" | {| class="wikitable" | ||
!Number||Use LB plate (+Amp) | !Number||Use LB plate (+Amp) | ||
| Line 98: | Line 86: | ||
|} | |} | ||
</div> | </div> | ||
| - | |||
===Liquid Culture=== | ===Liquid Culture=== | ||
| Line 113: | Line 100: | ||
===PCR=== | ===PCR=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">Hirano</span> | <span class="author">Hirano</span> | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !8/29 | + | !8/29 Pλ-RBS-luxI-DT||KOD plus||10x buffer||dNTP||MgSO4||F primer (RBS-luxI-DT-cloning-fwd) || R primer(RBS-luxI-DT-cloning-rev)||MilliQ||total |
|- | |- | ||
|0.3µL||0.5µL ||2.5µL ||2.5µL ||1.5µL ||0.75µL ||0.75µL ||16.2µL ||25µL | |0.3µL||0.5µL ||2.5µL ||2.5µL ||1.5µL ||0.75µL ||0.75µL ||16.2µL ||25µL | ||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{| class="wikitable" | {| class="wikitable" | ||
!8/31 Pbad/araC-RBS-RFP(1)||KOD plus||10x buffer||dNTP||MgSO4||F primer (Pbad/araC-pSB1C3-cloning-fond) ||R primer(Pbad/araC-cloning-rex)||MilliQ||total | !8/31 Pbad/araC-RBS-RFP(1)||KOD plus||10x buffer||dNTP||MgSO4||F primer (Pbad/araC-pSB1C3-cloning-fond) ||R primer(Pbad/araC-cloning-rex)||MilliQ||total | ||
| Line 143: | Line 115: | ||
!PreDenature||Denature||Annealing||Extension||cycle | !PreDenature||Denature||Annealing||Extension||cycle | ||
|- | |- | ||
| - | | | + | |94°C||98°C||65°C||68°C||-- |
|- | |- | ||
| - | |5min|| | + | |5min||10s||30s||3min10s||30cycles |
|} | |} | ||
</div> | </div> | ||
| - | |||
===Restriction Enzyme Digestion=== | ===Restriction Enzyme Digestion=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">No name</span> | <span class="author">No name</span> | ||
| Line 159: | Line 129: | ||
|2 cuts||10.5µL||1.0µL||1.0µL||3.0µL||0.3µL||14.2µL||30 µL | |2 cuts||10.5µL||1.0µL||1.0µL||3.0µL||0.3µL||14.2µL||30 µL | ||
|- | |- | ||
| - | |NC||0.5& | + | |NC||0.5µL||0µL||0µ||1.0µL||0.1µL||8.4µL||10 µL |
|} | |} | ||
| Line 167: | Line 137: | ||
|2 cuts||13.0µL||1.0µL||1.0µL||3.0µL||0.3µL||11.7µL||30 µL | |2 cuts||13.0µL||1.0µL||1.0µL||3.0µL||0.3µL||11.7µL||30 µL | ||
|- | |- | ||
| - | |NC||0.7& | + | |NC||0.7µL||0µL||0µ||1.0µL||0.1µL|| 8.2µL||10 µL |
|} | |} | ||
| Line 175: | Line 145: | ||
|2 cuts||9.0µL||1.0µL||1.0µL||3.0µL||0.3µL||15.7µL||30 µL | |2 cuts||9.0µL||1.0µL||1.0µL||3.0µL||0.3µL||15.7µL||30 µL | ||
|- | |- | ||
| - | |NC||0.5& | + | |NC||0.5µL||0µL||0µ||1.0µL||0.1µL|| 8.4µL||10 µL |
|} | |} | ||
| Line 183: | Line 153: | ||
|2 cuts||8.5µL||1.0µL||1.0µL||3.0µL||0.3µL||16.2µL||30 µL | |2 cuts||8.5µL||1.0µL||1.0µL||3.0µL||0.3µL||16.2µL||30 µL | ||
|- | |- | ||
| - | |NC||0.5& | + | |NC||0.5µL||0µL||0µ||1.0µL||0.1µL|| 8.4µL||10 µL |
|} | |} | ||
| Line 191: | Line 161: | ||
|2 cuts||5.4µL||1.0µL||1.0µL||3.0µL||3.0µL||16.6µL||30 µL | |2 cuts||5.4µL||1.0µL||1.0µL||3.0µL||3.0µL||16.6µL||30 µL | ||
|- | |- | ||
| - | |NC||0.3& | + | |NC||0.3µL||0µL||0µ||1.0µL||1.0µL|| 7.7µL||10 µL |
|} | |} | ||
| Line 199: | Line 169: | ||
|2 cuts||11µL||1.0µL||1.0µL||3.0µL||3.0µL||11µL||30 µL | |2 cuts||11µL||1.0µL||1.0µL||3.0µL||3.0µL||11µL||30 µL | ||
|- | |- | ||
| - | |NC||0.5& | + | |NC||0.5µL||0µL||0µ||1.0µL||1.0µL||7.5µL||10 µL |
|} | |} | ||
</div> | </div> | ||
| Line 213: | Line 183: | ||
|Ptrc KaiB||1µL||10µL||11µL||Amp | |Ptrc KaiB||1µL||10µL||11µL||Amp | ||
|- | |- | ||
| - | |pS& | + | |pSΩ ||1µL||10µL||11µL||Amp |
|} | |} | ||
</div> | </div> | ||
| Line 237: | Line 207: | ||
|6||100bp ladder||--||-- | |6||100bp ladder||--||-- | ||
|} | |} | ||
| - | + | ||
2 | 2 | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 254: | Line 224: | ||
|6||100bp ladder||--||-- | |6||100bp ladder||--||-- | ||
|} | |} | ||
| - | [[File: | + | |
| + | 3 | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||100bp ladder||--||-- | ||
| + | |- | ||
| + | |2||Spinach-DT||XbaI||PstI | ||
| + | |- | ||
| + | |3||Spinach-DT NC||--||-- | ||
| + | |- | ||
| + | |4||RBS-lacZα-DT||XbaI||PstI | ||
| + | |- | ||
| + | |5||RBS-lacα-DT NC||--||-- | ||
| + | |- | ||
| + | |6||100bp ladder||--||-- | ||
| + | |} | ||
| + | [[File:Igku_Sep4_Electrophoresis(N1).jpg]]<br> | ||
| + | [[File:Igku_Sep4_Electrophoresis_N2_3.jpg]]<br> | ||
</div> | </div> | ||
| Line 261: | Line 249: | ||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author">No name</span | + | <span class="author">No name</span> |
1 | 1 | ||
| Line 279: | Line 267: | ||
|- | |- | ||
|6||Plac||SpeI&PstI | |6||Plac||SpeI&PstI | ||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
2 | 2 | ||
| - | |||
{| class="wikitable" | {| class="wikitable" | ||
!Lane||DNA||Enzyme | !Lane||DNA||Enzyme | ||
| Line 313: | Line 284: | ||
|- | |- | ||
|6||RBS-tetR-DT||XbaI&PstI | |6||RBS-tetR-DT||XbaI&PstI | ||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | [[File: | + | [[File:Igku Sep4 Electrophoresis(N2).jpg]]<br> |
| - | [[File: | + | [[File:Igku Sep4 Gel Extraction(N2) 2.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Name||quantity||concentration[µg/mL]||260/280||260/230 | !Name||quantity||concentration[µg/mL]||260/280||260/230 | ||
|- | |- | ||
| - | |Ptet||-- ||30.1 ||1.97 ||-0.97 | + | |Pcon||--||13.2 ||1.99 ||-1.64 |
| + | |- | ||
| + | |Plac||-- ||15.3 ||2.07 ||-0.39 | ||
| + | |- | ||
| + | |Ptet||-- ||30.1||1.97 ||-0.97 | ||
|- | |- | ||
| - | |RBS-tetR-DT||-- ||3.0 ||2.44 ||0.88 | + | |RBS-tetR-DT||--||3.0||2.44 ||0.88 |
|} | |} | ||
| + | </div> | ||
===Gel Extraction=== | ===Gel Extraction=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">No name</span></div> | <span class="author">No name</span></div> | ||
| - | |||
| - | |||
{| class="wikitable" | {| class="wikitable" | ||
!Lane||DNA||Enzyme | !Lane||DNA||Enzyme | ||
| Line 339: | Line 308: | ||
|1||100bp ladder||-- | |1||100bp ladder||-- | ||
|- | |- | ||
| - | |2||spinach-DT|| | + | |2||spinach-DT||XbaI&PstI |
|- | |- | ||
| - | |3||spinach-DT | + | |3||spinach-DT ||XbaI&PstI |
|- | |- | ||
| - | |4|| | + | |4||--||-- |
|- | |- | ||
| - | |5||RBS-lacZα-DT | + | |5||RBS-lacZα-DT||XbaI&PstI |
|- | |- | ||
| - | |6|| | + | |6||RBS-lacZα-DT||XbaI&PstI |
|} | |} | ||
| - | [[File: | + | [[File:Igku Sep4 Gel Extraction(N3) 1.jpg]]<br> |
| - | [[File: | + | [[File:Igku Sep4 Gel Extraction(N3) 2.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Name||quantity||concentration[µg/mL]||260/280||260/230 | !Name||quantity||concentration[µg/mL]||260/280||260/230 | ||
|- | |- | ||
| - | |spinach-DT||--||2.1 ||2.10 ||-0.05 | + | |spinach-DT||--||2.1||2.10||-0.05 |
|- | |- | ||
| - | |RBS-lacZα-DT||-- ||3.3 ||2.59 ||-0.07 | + | |RBS-lacZα-DT||--||3.3||2.59||-0.07 |
|} | |} | ||
</div> | </div> | ||
| Line 373: | Line 342: | ||
|- | |- | ||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{| class="wikitable" | {| class="wikitable" | ||
!Number||Use LB plate | !Number||Use LB plate | ||
| Line 389: | Line 352: | ||
|- | |- | ||
|4||JM109-4 | |4||JM109-4 | ||
| - | |||
|} | |} | ||
| - | |||
| - | |||
| - | |||
</div> | </div> | ||
| Line 403: | Line 362: | ||
|- | |- | ||
|8/9 BBa_J23100-3||Plusgrow medium(+Amp) | |8/9 BBa_J23100-3||Plusgrow medium(+Amp) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|- | |- | ||
|JM109-1(Master plate)||LB medium | |JM109-1(Master plate)||LB medium | ||
| - | |||
|} | |} | ||
</div> | </div> | ||
| Line 437: | Line 386: | ||
|- | |- | ||
|5min||30s||30s||30s||30cycles | |5min||30s||30s||30s||30cycles | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
</div> | </div> | ||
Latest revision as of 00:34, 27 September 2013
Contents |
Sep 4
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | 9/3 DT(EcoRI&XbaI) | 3.2µL | 9/3 RBS-lysis1 | 2.4µL | 2.7µL |
| experiment | 9/3 DT(EcoRI&XbaI) | 3.0µL | 9/3 RBS-lysis2 | 4.7µL | 4.0µL |
| experiment | 9/3 DT(EcoRI&XbaI) | 3.0µL | 9/3 RBS-lysis3 | 8.8µL | 4.0µL |
| experiment | 9/3 DT(EcoRI&XbaI) | 3.0µL | 9/3 aptamer12_1R(EcoRI&SpeI) | 1.9µL | 2.45µL |
| experiment | 9/2 pSB1C3(XbaI&PstI) | 10.6µL | 9/3 pT181attenuator (XbaI& PstI) | 3.2µL | 4.0µL |
| experiment | 9/2 pSB1C3(XbaI&PstI) | 9.6µL | 9/3 pT181antisense (XbaI& PstI) | 2.1µL | 4.0µL |
| experiment | 9/2 pSB1C3(EcoRI&SpeI) | 16.7µL | 9/3 aptamer12_1R(EcoRI&SpeI) | 2.0µL | 4.0µL |
incubate 16 °C 1 hour
Transformation
| Name | Sample | Competent Cells | Plate |
|---|---|---|---|
| 9/4 RBS-lysis1-DT | 2µL | 20µL | Amp |
| 9/4 RBS-lysis2-DT | 2µL | 20µL | Amp |
| 9/4 RBS-lysis3-DT | 2µL | 20µL | Amp |
| 9/4 aptamer12_1R-DT | 2µL | 20µL | Amp |
| 9/4 pT181attenuator –pSB1C3 | 2µL | 20µL | Amp |
| 9/4 pT181antisense-pSB1C3 | 2µL | 20µL | Amp |
| 9/4 aptamer12_1R –pSB1C3 | 2µL | 20µL | Amp |
| 9/4 pSB4K5(2013plate5 56) | 2µL | 20µL | Amp |
| 9/3 Mutagenesis product 1(KaiABC) | 2µL | 20µL | Amp |
| 9/3 Mutagenesis product 2(NC) | 2µL | 20µL | Amp |
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| DT | 193.1 | 1.93 | 1.81 |
| Spinach-DT | 373.3 | 1.92 | 1.69 |
| RBS-lysis1 | 332.0 | 1.95 | 1.62 |
Master Plate
| Number | Use LB plate (+CP) |
|---|---|
| 1 | 8/10 Plac(CP)-2 |
| 2 | 8/10 Plac(CP)-2 |
| 3 | 8/10 Plac(CP)-2 |
| Number | Use LB plate (+Amp) |
|---|---|
| 1 | 8/9 J23100(Amp)-1 |
| 2 | 8/9 J23100(Amp)-1 |
| 3 | 8/9 J23100(Amp)-1 |
Liquid Culture
| Sample | medium |
|---|---|
| 8/10 Plac(CP)-2 | Plusgrow medium(+CP) |
| 8/9 J23100(Amp)-1 | Plusgrow medium(+Amp) |
PCR
| 8/29 Pλ-RBS-luxI-DT | KOD plus | 10x buffer | dNTP | MgSO4 | F primer (RBS-luxI-DT-cloning-fwd) | R primer(RBS-luxI-DT-cloning-rev) | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.3µL | 0.5µL | 2.5µL | 2.5µL | 1.5µL | 0.75µL | 0.75µL | 16.2µL | 25µL |
| 8/31 Pbad/araC-RBS-RFP(1) | KOD plus | 10x buffer | dNTP | MgSO4 | F primer (Pbad/araC-pSB1C3-cloning-fond) | R primer(Pbad/araC-cloning-rex) | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.3µL | 0.5µL | 2.5µL | 2.5µL | 1.5µL | 0.75µL | 0.75µL | 16.2µL | 25µL |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 65°C | 68°C | -- |
| 5min | 10s | 30s | 3min10s | 30cycles |
Restriction Enzyme Digestion
| 8/21 Pconst | SpeI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 10.5µL | 1.0µL | 1.0µL | 3.0µL | 0.3µL | 14.2µL | 30 µL |
| NC | 0.5µL | 0µL | 0µ | 1.0µL | 0.1µL | 8.4µL | 10 µL |
| 8/29 Plac | SpeI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 13.0µL | 1.0µL | 1.0µL | 3.0µL | 0.3µL | 11.7µL | 30 µL |
| NC | 0.7µL | 0µL | 0µ | 1.0µL | 0.1µL | 8.2µL | 10 µL |
| 8/20 Ptet | SpeI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 9.0µL | 1.0µL | 1.0µL | 3.0µL | 0.3µL | 15.7µL | 30 µL |
| NC | 0.5µL | 0µL | 0µ | 1.0µL | 0.1µL | 8.4µL | 10 µL |
| 8/20 RBS-tetR-DT | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 8.5µL | 1.0µL | 1.0µL | 3.0µL | 0.3µL | 16.2µL | 30 µL |
| NC | 0.5µL | 0µL | 0µ | 1.0µL | 0.1µL | 8.4µL | 10 µL |
| spinach -DT | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 5.4µL | 1.0µL | 1.0µL | 3.0µL | 3.0µL | 16.6µL | 30 µL |
| NC | 0.3µL | 0µL | 0µ | 1.0µL | 1.0µL | 7.7µL | 10 µL |
| 8/17 RBS-lacZα-DT | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 11µL | 1.0µL | 1.0µL | 3.0µL | 3.0µL | 11µL | 30 µL |
| NC | 0.5µL | 0µL | 0µ | 1.0µL | 1.0µL | 7.5µL | 10 µL |
Transformation
| Name | Sample | Competent Cells | Total | Plate |
|---|---|---|---|---|
| Ptrc KaiC | 1µL | 10µL | 11µL | Amp |
| Ptrc KaiB | 1µL | 10µL | 11µL | Amp |
| pSΩ | 1µL | 10µL | 11µL | Amp |
Electrophoresis
1
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | Pconst | SpeI | PstI |
| 3 | Pconst NC | -- | -- |
| 4 | Plac | SpeI | PstI |
| 5 | Plac NC | -- | -- |
| 6 | 100bp ladder | -- | -- |
2
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | Ptet | SpeI | PstI |
| 3 | Ptet NC | -- | -- |
| 4 | RBS-tetR-DT | XbaI | PstI |
| 5 | RBS-tetR-DT NC | -- | -- |
| 6 | 100bp ladder | -- | -- |
3
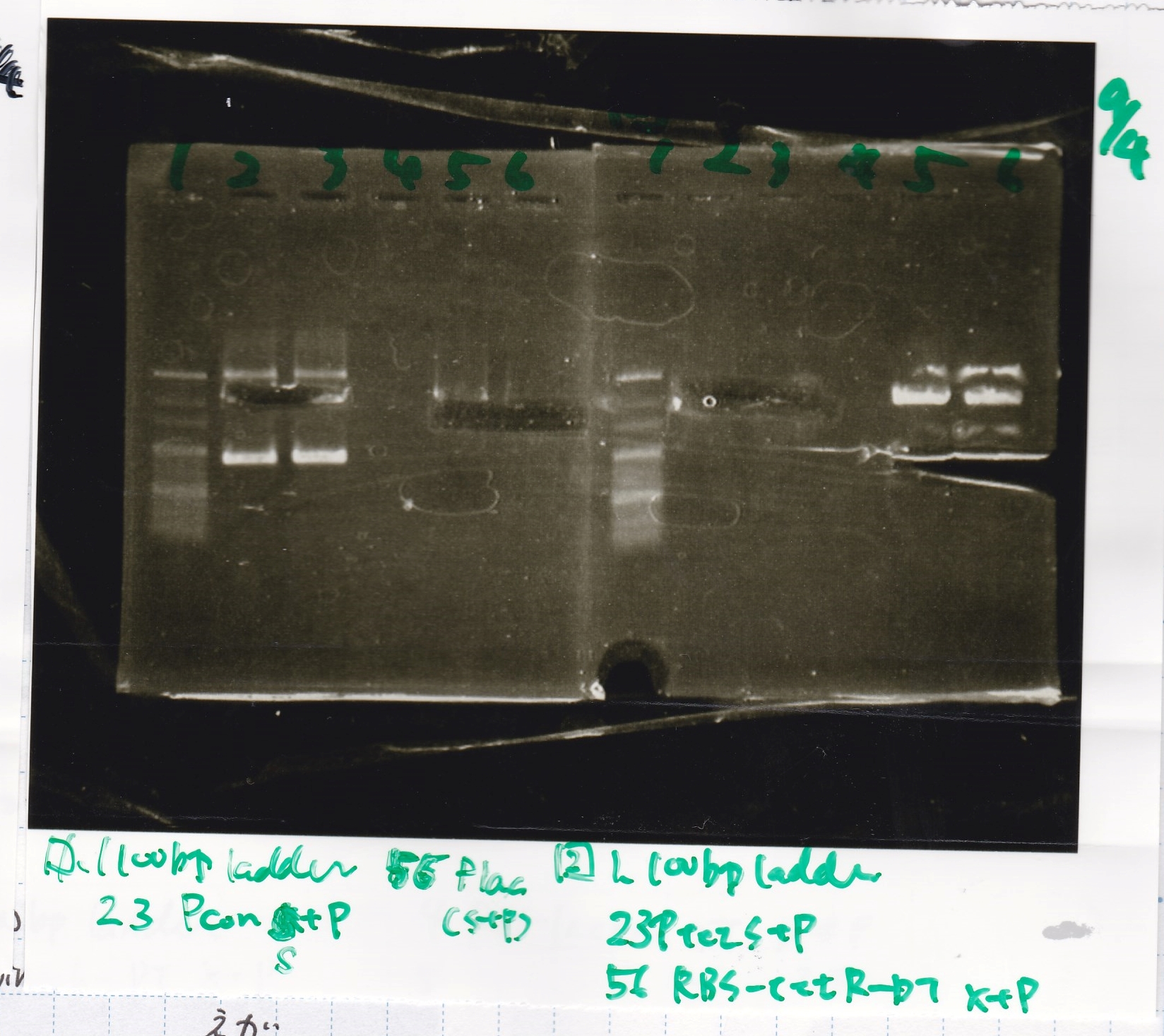
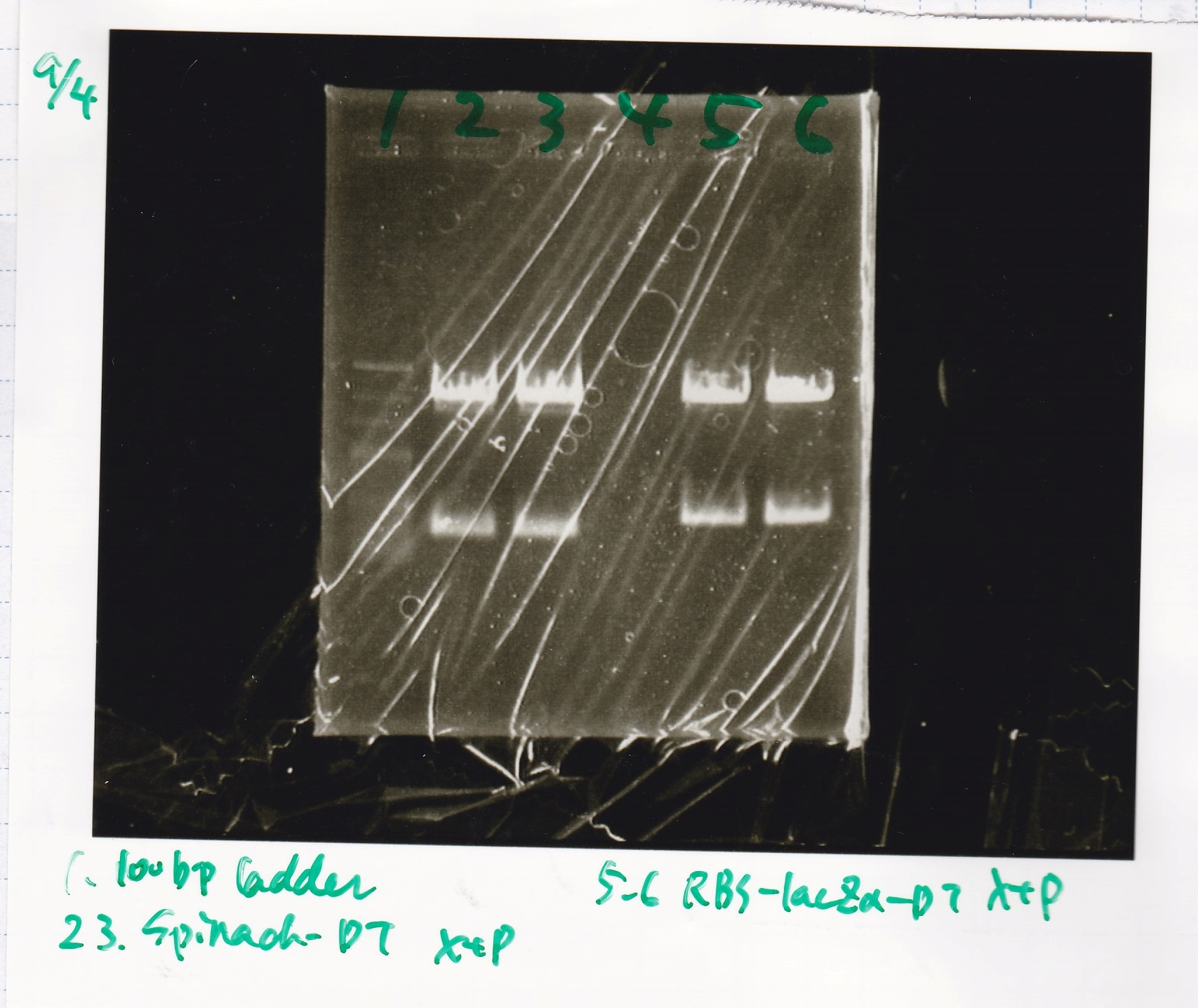
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | Spinach-DT | XbaI | PstI |
| 3 | Spinach-DT NC | -- | -- |
| 4 | RBS-lacZα-DT | XbaI | PstI |
| 5 | RBS-lacα-DT NC | -- | -- |
| 6 | 100bp ladder | -- | -- |
Gel Extraction
1
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 2 | Pcon | SpeI&PstI |
| 3 | Pcon | SpeI&PstI |
| 4 | -- | -- |
| 5 | Plac | SpeI&PstI |
| 6 | Plac | SpeI&PstI |
2
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 2 | Ptet | SpeI&PstI |
| 3 | Ptet | SpeI&PstI |
| 4 | -- | -- |
| 5 | RBS-tetR-DT | XbaI&PstI |
| 6 | RBS-tetR-DT | XbaI&PstI |
| Name | quantity | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|---|
| Pcon | -- | 13.2 | 1.99 | -1.64 |
| Plac | -- | 15.3 | 2.07 | -0.39 |
| Ptet | -- | 30.1 | 1.97 | -0.97 |
| RBS-tetR-DT | -- | 3.0 | 2.44 | 0.88 |
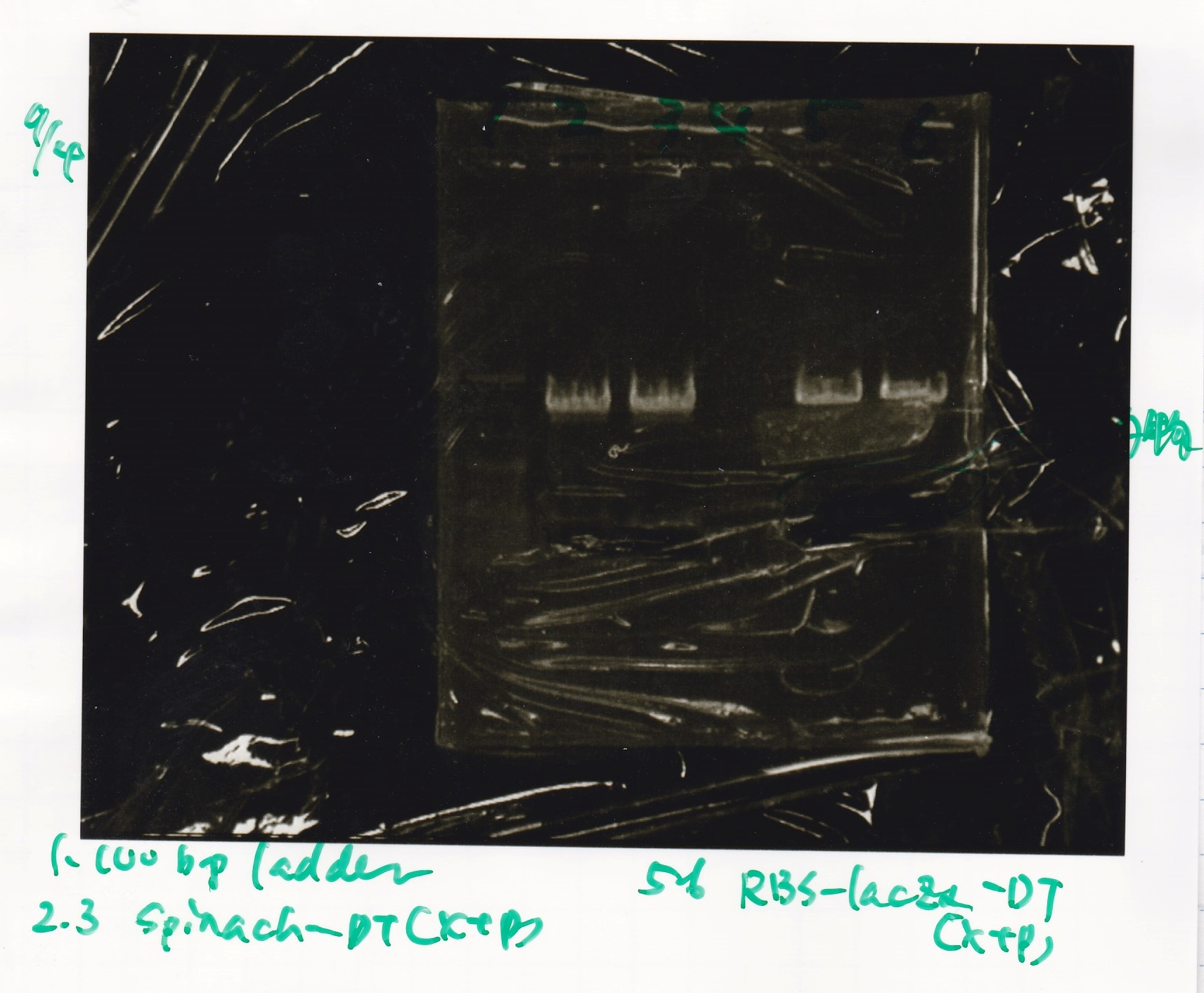
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | -- |
| 2 | spinach-DT | XbaI&PstI |
| 3 | spinach-DT | XbaI&PstI |
| 4 | -- | -- |
| 5 | RBS-lacZα-DT | XbaI&PstI |
| 6 | RBS-lacZα-DT | XbaI&PstI |
| Name | quantity | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|---|
| spinach-DT | -- | 2.1 | 2.10 | -0.05 |
| RBS-lacZα-DT | -- | 3.3 | 2.59 | -0.07 |
Master Plate
| Number | Use LB plate (+Amp) |
|---|---|
| 4 | 8/9 BBa_J23100-3 |
| 5 | 8/9 BBa_J23100-4 |
| 6 | 8/9 BBa_J23100-5 |
| Number | Use LB plate |
|---|---|
| 1 | JM109-1 |
| 2 | JM109-2 |
| 3 | JM109-3 |
| 4 | JM109-4 |
Liquid Culture
| Sample | medium |
|---|---|
| 8/9 BBa_J23100-3 | Plusgrow medium(+Amp) |
| JM109-1(Master plate) | LB medium |
Colony PCR
| Sample | base pair |
|---|---|
| 8/9 BBa_J23100 3 | -- |
| 8/9 BBa_J23100 4 | -- |
| 8/9 BBa_J23100 5 | -- |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 30s | 30cycles |
 "
"