Template:Kyoto/Notebook/Sep 10
From 2013.igem.org
(Difference between revisions)
(→Miniprep) |
(→Gel Extraction) |
||
| (15 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
==Sep 10== | ==Sep 10== | ||
===Ligation=== | ===Ligation=== | ||
| - | |||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">Nakamoto</span> | <span class="author">Nakamoto</span> | ||
| Line 8: | Line 6: | ||
!state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | !state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | ||
|- | |- | ||
| - | |experiment||9/6 Pcon-RBS-luxR-DT||1.9µL||9/6Plux-RBS-GFP-DT||22 µL|| µL | + | |experiment||9/6 Pcon-RBS-luxR-DT||1.9µL||9/6Plux-RBS-GFP-DT||22 µL||3.5µL |
|- | |- | ||
| - | |experiment||9/6 Plux||2µL||9/8 RBS-lysis1-DT||6.6µL|| | + | |experiment||9/6 Plux||2µL||9/8 RBS-lysis1-DT||6.6µL||3.5µL |
|- | |- | ||
| - | |experiment||9/6 Plux||2µL||9/8 RBS-lysis2-DT||6.3µL|| | + | |experiment||9/6 Plux||2µL||9/8 RBS-lysis2-DT||6.3µL||3.5µL |
|- | |- | ||
| - | |experiment||9/8 Ptet-PT181 antisense||1.6µL||9/8 spinach-DT||4.4µL|| | + | |experiment||9/8 Ptet-PT181 antisense||1.6µL||9/8 spinach-DT||4.4µL||3.0µL |
|- | |- | ||
| - | |experiment||9/8 Pcon-PT181 attenuator||3.4µL||9/8 apz 12-1R-PT||2.1µL|| | + | |experiment||9/8 Pcon-PT181 attenuator||3.4µL||9/8 apz 12-1R-PT||2.1µL||2.8µL |
|- | |- | ||
| - | |experiment||9/8 Pcon||3.4µL||9/8 apz 12-1R-PT||2.3µL|| | + | |experiment||9/8 Pcon||3.4µL||9/8 apz 12-1R-PT||2.3µL||2,9µL |
|- | |- | ||
| - | |experiment||9/8 DT||3.0µL||9/8 Pcon-PT181 attenuator||7.9µL|| | + | |experiment||9/8 DT||3.0µL||9/8 Pcon-PT181 attenuator||7.9µL||3.5µL |
|- | |- | ||
| - | |experiment||9/10 pSB1C3||1.8µL||9/8 F3m2 attenuator||4.6µL|| | + | |experiment||9/10 pSB1C3||1.8µL||9/8 F3m2 attenuator||4.6µL||3.2µL |
|- | |- | ||
| - | |experiment||9/10 pSB1C3||1.8µL||9/8 F1 attenuator||3.4µL|| | + | |experiment||9/10 pSB1C3||1.8µL||9/8 F1 attenuator||3.4µL||2.6µL |
|} | |} | ||
</div> | </div> | ||
| - | |||
===Miniprep=== | ===Miniprep=== | ||
| Line 48: | Line 45: | ||
===Restriction Enzyme Digestion=== | ===Restriction Enzyme Digestion=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">Nakamoto</span> | <span class="author">Nakamoto</span> | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||9/10 Plux-RBS-GFP-DT|| | + | ! ||9/10 Plux-RBS-GFP-DT||EcoRI||SpeI||Buffer||BSA||MilliQ||total |
|- | |- | ||
| - | |2cuts||1µL||1µL||3µL||3µL||9.9µL||30µL | + | |2cuts||12.1µL||1µL||1µL||3µL||3µL||9.9µL||30µL |
|- | |- | ||
| - | |NC|| 0µL||0µL||1µL||1µL||7.4µL||10µL | + | |NC||0.6µL||0µL||0µL||1µL||1µL||7.4µL||10µL |
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||9/10 Spinach-DT|| | + | ! ||9/10 Spinach-DT||XbaI||PstI||Buffer||BSA||MilliQ||total |
|- | |- | ||
| - | |2cuts||1µL||1µL||3µL||3µL||12.1µL||30µL | + | |2cuts||9.9µL||1µL||1µL||3µL||3µL||12.1µL||30µL |
|- | |- | ||
| - | |NC|| 0µL||0µL||1µL||1µL||7.5µL||10µL | + | |NC||0.5µL||0µL||0µL||1µL||1µL||7.5µL||10µL |
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||9/6 apt12-1R -DT|| | + | ! ||9/6 apt12-1R -DT||XbaI||PstI||Buffer||BSA||MilliQ||total |
|- | |- | ||
| - | |2cuts||1µL||1µL||3µL||3µL||14.0µL||30µL | + | |2cuts||8.0µL||1µL||1µL||3µL||3µL||14.0µL||30µL |
|- | |- | ||
| - | |NC|| 0µL||0µL||1µL||1µL||7.6µL||10µL | + | |NC||0.4µL||0µL||0µL||1µL||1µL||7.6µL||10µL |
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||9/10 Pcon-pt81 attenuator || | + | ! ||9/10 Pcon-pt81 attenuator ||EcoRI||SpeI||Buffer||BSA||MilliQ||total |
|- | |- | ||
| - | |2cuts||1µL||1µL||3µL||3µL||12.2µL||30µL | + | |2cuts||9.8µL||1µL||1µL||3µL||3µL||12.2µL||30µL |
|- | |- | ||
| - | |NC|| 0µL||0µL||1µL||1µL||7.5µL||10µL | + | |NC||0.5µL||0µL||0µL||1µL||1µL||7.5µL||10µL |
|} | |} | ||
</div> | </div> | ||
| - | |||
===Electrophoresis=== | ===Electrophoresis=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">No name</span> |
{| class="wikitable" | {| class="wikitable" | ||
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
| Line 92: | Line 86: | ||
|1||100bp ladder||--||-- | |1||100bp ladder||--||-- | ||
|- | |- | ||
| - | |2|| Plux-RBS-GEP-DT|| | + | |2|| Plux-RBS-GEP-DT||EcoRI||SpeI |
|- | |- | ||
|3|| Plux-RBS-GEP-DT||--||-- | |3|| Plux-RBS-GEP-DT||--||-- | ||
|- | |- | ||
| - | |4||Spinach-DT|| | + | |4||Spinach-DT||XbaI||PstI |
|- | |- | ||
|5||Spinach-DT||--||-- | |5||Spinach-DT||--||-- | ||
|- | |- | ||
| - | |6||aptamer 121R-DT|| | + | |6||aptamer 121R-DT||XbaI||PstI |
|- | |- | ||
|7||aptamer 121R-DT||--||-- | |7||aptamer 121R-DT||--||-- | ||
|- | |- | ||
| - | |8||Pcon-pt181 attenuator||| | + | |8||Pcon-pt181 attenuator|||EcoRI||SpeI |
|- | |- | ||
|9|| Pcon-pt181 attenuator||--||-- | |9|| Pcon-pt181 attenuator||--||-- | ||
| Line 110: | Line 104: | ||
|10||100bp ladder||--||-- | |10||100bp ladder||--||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_9104.jpg]]<br> |
</div> | </div> | ||
| - | |||
| - | |||
===Colony PCR=== | ===Colony PCR=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">Tatsui</span> | <span class="author">Tatsui</span> | ||
| Line 131: | Line 122: | ||
|94°C||94°C||55°C||68°C||-- | |94°C||94°C||55°C||68°C||-- | ||
|- | |- | ||
| - | |5min||30s||30s||1min10sec|| | + | |5min||30s||30s||1min10sec||30 |
|} | |} | ||
</div> | </div> | ||
| - | |||
===Electrophoresis=== | ===Electrophoresis=== | ||
| - | + | <div class="experiment"> | |
| - | + | ||
| - | <div class="experiment"> | + | |
<span class="author">Tatsui</span> | <span class="author">Tatsui</span> | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 172: | Line 160: | ||
|14||100bp ladder | |14||100bp ladder | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_0910_E2.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Lane||Sample | !Lane||Sample | ||
| Line 187: | Line 175: | ||
|} | |} | ||
</div> | </div> | ||
| - | + | [[File:Igku_0910_E3.jpg]] | |
===Gel Extraction=== | ===Gel Extraction=== | ||
| Line 216: | Line 204: | ||
|10||1kbp ladder||-- | |10||1kbp ladder||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_9101.jpg]]<br> |
| - | [[File: | + | [[File:igku_9102.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Lane||DNA||Enzyme | !Lane||DNA||Enzyme | ||
| Line 242: | Line 230: | ||
|} | |} | ||
</div> | </div> | ||
| - | + | [[File:Igku_0910_G3.jpg]] | |
===Liquid Culture=== | ===Liquid Culture=== | ||
| Line 259: | Line 247: | ||
===Gel Extraction=== | ===Gel Extraction=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">Tatsui</span> | <span class="author">Tatsui</span> | ||
| Line 284: | Line 271: | ||
|- | |- | ||
|10||KaiC||-- | |10||KaiC||-- | ||
| + | |- | ||
|11||--||-- | |11||--||-- | ||
|- | |- | ||
|12||1kbp ladder||-- | |12||1kbp ladder||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_9105.jpg]]<br> |
| - | [[File: | + | [[File:igku_9106.jpg]]<br> |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
</div> | </div> | ||
| - | |||
===Liquid Culture=== | ===Liquid Culture=== | ||
| Line 313: | Line 294: | ||
===Colony PCR=== | ===Colony PCR=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">No name</span> | <span class="author">No name</span> | ||
| Line 326: | Line 306: | ||
|94°C||94°C||55°C||68°C||-- | |94°C||94°C||55°C||68°C||-- | ||
|- | |- | ||
| - | |5min||30s||30s||2min|| | + | |5min||30s||30s||2min||30 |
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 340: | Line 320: | ||
|94°C||94°C||55°C||68°C||-- | |94°C||94°C||55°C||68°C||-- | ||
|- | |- | ||
| - | |5min||30s||30s||42s|| | + | |5min||30s||30s||42s||30 |
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 354: | Line 334: | ||
|94°C||94°C||55°C||68°C||-- | |94°C||94°C||55°C||68°C||-- | ||
|- | |- | ||
| - | |5min||30s||30s||1min12s|| | + | |5min||30s||30s||1min12s||30 |
|} | |} | ||
</div> | </div> | ||
| - | |||
===Electrophoresis=== | ===Electrophoresis=== | ||
| Line 380: | Line 359: | ||
|6||100bp ladder||--||-- | |6||100bp ladder||--||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_91014.jpg]]<br> |
</div> | </div> | ||
<!-- ここまでをコピーしてね --> | <!-- ここまでをコピーしてね --> | ||
| + | |||
===Transformation=== | ===Transformation=== | ||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">Nakamoto</span> | <span class="author">Nakamoto</span> | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !Name | + | !Name||Plate |
|- | |- | ||
| - | |F1 attenuator +p3B1C3 | + | |F1 attenuator +p3B1C3||CP |
|- | |- | ||
| - | |F3n2 attenuator +p3B1C3 | + | |F3n2 attenuator +p3B1C3||CP |
|- | |- | ||
| - | |F1 antisense +p3B1C3 | + | |F1 antisense +p3B1C3||CP |
|- | |- | ||
| - | |F6 antisense+p3B1C3 | + | |F6 antisense+p3B1C3||CP |
|- | |- | ||
| - | |apt12-P +p3B1C3 | + | |apt12-P +p3B1C3||CP |
|- | |- | ||
| - | |apt12-1M +p3B1C3 | + | |apt12-1M +p3B1C3||CP |
|- | |- | ||
| - | |Pcon-luxR+Plux-GFP | + | |Pcon-luxR+Plux-GFP||Amp |
|- | |- | ||
| - | |Plux+RBS-lysis1-DT | + | |Plux+RBS-lysis1-DT||CP |
|- | |- | ||
| - | |Plux+RBS-lysis2-DT | + | |Plux+RBS-lysis2-DT||CP |
|- | |- | ||
| - | |Ptet-pT181antisense+Spinach-DT | + | |Ptet-pT181antisense+Spinach-DT||CP |
|- | |- | ||
| - | |Pcon-pT181 attenuator+apt121R-DT | + | |Pcon-pT181 attenuator+apt121R-DT||Amp |
|- | |- | ||
| - | |Pcon-1 apt12-1R-DT | + | |Pcon-1 apt12-1R-DT||Amp |
|- | |- | ||
| - | |DT+Pcon-pT181 attenuator | + | |DT+Pcon-pT181 attenuator||CP |
|} | |} | ||
</div> | </div> | ||
| - | |||
===Electrophoresis=== | ===Electrophoresis=== | ||
| Line 428: | Line 407: | ||
|1||100bp ladder||--||-- | |1||100bp ladder||--||-- | ||
|- | |- | ||
| - | |2||Spinach-DT|| | + | |2||Spinach-DT||XbaI||PstI |
|- | |- | ||
| - | |3||Spinach-DT|| | + | |3||Spinach-DT||XbaI||PstI |
|- | |- | ||
| - | |4||Spinach-DT|| | + | |4||Spinach-DT||XbaI||PstI |
|- | |- | ||
| - | |5||Spinach-DT|| | + | |5||Spinach-DT||XbaI||PstI |
|- | |- | ||
|6||100bp ladder||--||-- | |6||100bp ladder||--||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_9108.jpg]]<br> |
| + | [[File:igku_9109.jpg]]<br> | ||
{| class="wikitable" | {| class="wikitable" | ||
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
| Line 444: | Line 424: | ||
|1||100bp ladder||--||-- | |1||100bp ladder||--||-- | ||
|- | |- | ||
| - | |2||Pcon-pT181 attenuator|| | + | |2||Pcon-pT181 attenuator||EcoRI||SpeI |
|- | |- | ||
| - | |3||Pcon-pT181 attenuator|| | + | |3||Pcon-pT181 attenuator||EcoRI||SpeI |
|- | |- | ||
| - | |4||Pcon-pT181 attenuator|| | + | |4||Pcon-pT181 attenuator||EcoRI||Spei |
|- | |- | ||
| - | |5||Pcon-pT181 attenuator|| | + | |5||Pcon-pT181 attenuator||EcoRI||SpeI |
|- | |- | ||
|6||100bp ladder||--||-- | |6||100bp ladder||--||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_91010.jpg]]<br> |
| + | [[File:igku_91011.jpg]]<br> | ||
{| class="wikitable" | {| class="wikitable" | ||
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
|- | |- | ||
| - | |1||Plux-RBS-GFP-DT|| | + | |1||Plux-RBS-GFP-DT||EcoRI||SpeI |
|- | |- | ||
| - | |2||Plux-RBS-GFP-DT || | + | |2||Plux-RBS-GFP-DT ||EcoRI||SpeI |
|- | |- | ||
| - | |3||Plux-RBS-GFP-DT || | + | |3||Plux-RBS-GFP-DT ||EcoRI||SpeI |
|- | |- | ||
| - | |4||Plux-RBS-GFP-DT || | + | |4||Plux-RBS-GFP-DT ||EcoRI||SpeI |
|- | |- | ||
|5||100bp ladder||--||-- | |5||100bp ladder||--||-- | ||
|- | |- | ||
| - | |6||apt12-1R-DT|| | + | |6||apt12-1R-DT||XbaI||PstI |
|- | |- | ||
| - | |7||apt12-1R-DT|| | + | |7||apt12-1R-DT||XbaI||PstI |
|- | |- | ||
| - | |8||apt12-1R-DT|| | + | |8||apt12-1R-DT||XbaI||PstI |
|- | |- | ||
| - | |9||apt12-1R-DT|| | + | |9||apt12-1R-DT||XbaI||PstI |
|} | |} | ||
| - | [[File: | + | [[File:igku_91012.jpg]]<br> |
| - | + | [[File:igku_91013.jpg]]<br> | |
</div> | </div> | ||
<!-- ここまでをコピーしてね --> | <!-- ここまでをコピーしてね --> | ||
Latest revision as of 18:06, 27 September 2013
Contents |
Sep 10
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | 9/6 Pcon-RBS-luxR-DT | 1.9µL | 9/6Plux-RBS-GFP-DT | 22 µL | 3.5µL |
| experiment | 9/6 Plux | 2µL | 9/8 RBS-lysis1-DT | 6.6µL | 3.5µL |
| experiment | 9/6 Plux | 2µL | 9/8 RBS-lysis2-DT | 6.3µL | 3.5µL |
| experiment | 9/8 Ptet-PT181 antisense | 1.6µL | 9/8 spinach-DT | 4.4µL | 3.0µL |
| experiment | 9/8 Pcon-PT181 attenuator | 3.4µL | 9/8 apz 12-1R-PT | 2.1µL | 2.8µL |
| experiment | 9/8 Pcon | 3.4µL | 9/8 apz 12-1R-PT | 2.3µL | 2,9µL |
| experiment | 9/8 DT | 3.0µL | 9/8 Pcon-PT181 attenuator | 7.9µL | 3.5µL |
| experiment | 9/10 pSB1C3 | 1.8µL | 9/8 F3m2 attenuator | 4.6µL | 3.2µL |
| experiment | 9/10 pSB1C3 | 1.8µL | 9/8 F1 attenuator | 3.4µL | 2.6µL |
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| spinach-DT | 202.4 | 1.97 | 1.93 |
| Plux-RBS-GFP-DT | 164.2 | 2.80 | 2.06 |
| Pcon-DT attenuator | 205.4 | 1.81 | 2.39 |
| RSBJC3-2 | 240.8 | 1.73 | 1.43 |
| Plaz-1 | 240.8 | 1.73 | 1.43 |
Restriction Enzyme Digestion
| 9/10 Plux-RBS-GFP-DT | EcoRI | SpeI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2cuts | 12.1µL | 1µL | 1µL | 3µL | 3µL | 9.9µL | 30µL |
| NC | 0.6µL | 0µL | 0µL | 1µL | 1µL | 7.4µL | 10µL |
| 9/10 Spinach-DT | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2cuts | 9.9µL | 1µL | 1µL | 3µL | 3µL | 12.1µL | 30µL |
| NC | 0.5µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL |
| 9/6 apt12-1R -DT | XbaI | PstI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2cuts | 8.0µL | 1µL | 1µL | 3µL | 3µL | 14.0µL | 30µL |
| NC | 0.4µL | 0µL | 0µL | 1µL | 1µL | 7.6µL | 10µL |
| 9/10 Pcon-pt81 attenuator | EcoRI | SpeI | Buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2cuts | 9.8µL | 1µL | 1µL | 3µL | 3µL | 12.2µL | 30µL |
| NC | 0.5µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL |
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | Plux-RBS-GEP-DT | EcoRI | SpeI |
| 3 | Plux-RBS-GEP-DT | -- | -- |
| 4 | Spinach-DT | XbaI | PstI |
| 5 | Spinach-DT | -- | -- |
| 6 | aptamer 121R-DT | XbaI | PstI |
| 7 | aptamer 121R-DT | -- | -- |
| 8 | Pcon-pt181 attenuator | EcoRI | SpeI |
| 9 | Pcon-pt181 attenuator | -- | -- |
| 10 | 100bp ladder | -- | -- |
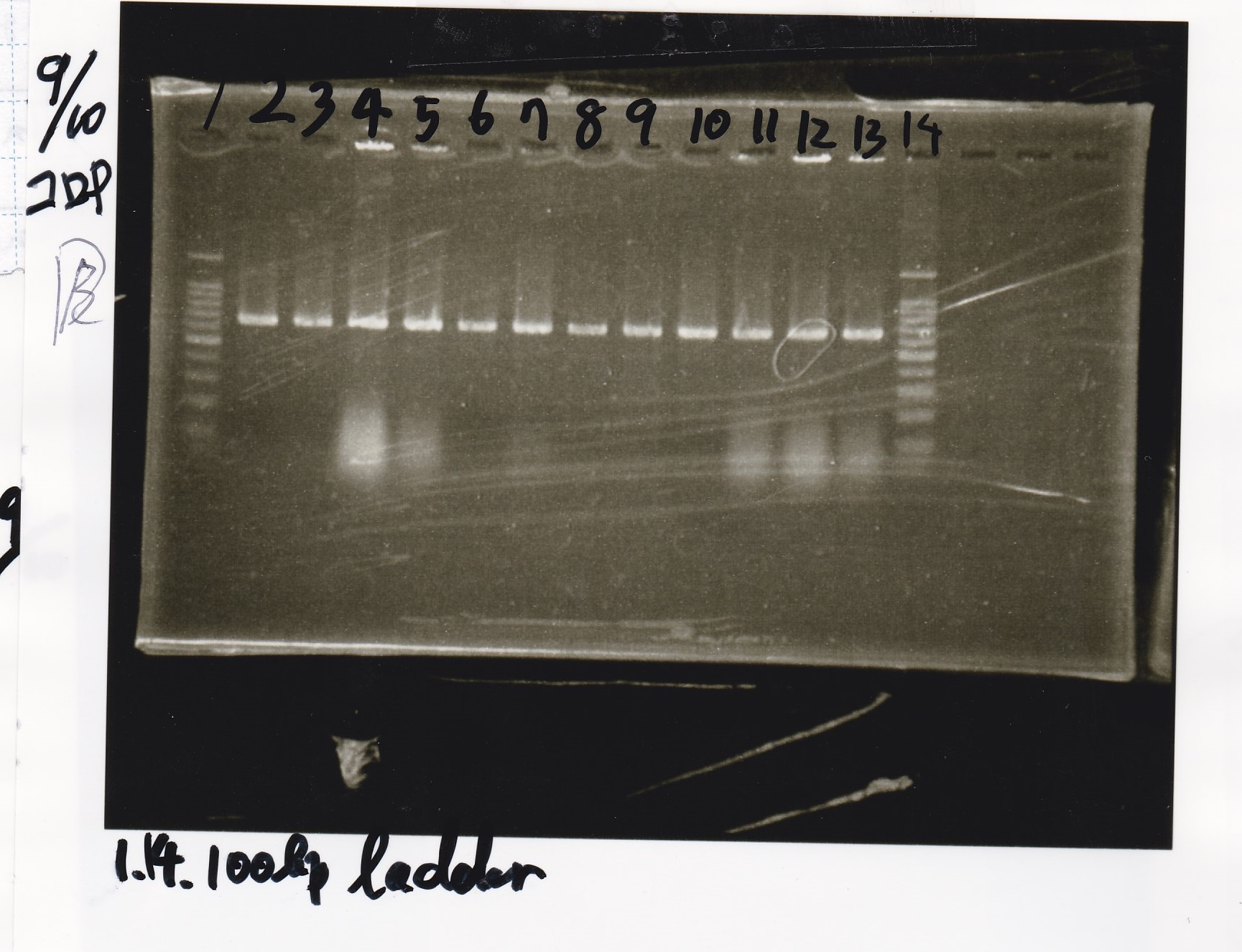
Colony PCR
| Sample | base pair |
|---|---|
| 9/9 Pcon-pT181 antisense | -- |
| 9/8 J223100(entA) | -- |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 1min10sec | 30 |
Electrophoresis
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | Pcon Pt181 antisense1+spinach-DT |
| 3 | Pcon Pt181 antisense2+spinach-DT |
| 4 | Pcon Pt181 antisense3+spinach-DT |
| 5 | Pcon Pt181 antisense4+spinach-DT |
| 6 | Pcon Pt181 antisense5+spinach-DT |
| 7 | Pcon Pt181 antisense6+spinach-DT |
| 8 | Pcon Pt181 antisense7+spinach-DT |
| 9 | Pcon Pt181 antisense8+spinach-DT |
| 10 | Pcon Pt181 antisense9+spinach-DT |
| 11 | Pcon Pt181 antisense10+spinach-DT |
| 12 | Pcon Pt181 antisense11+spinach-DT |
| 13 | Pcon Pt181 antisense12+spinach-DT |
| 14 | 100bp ladder |
| Lane | Sample |
|---|---|
| 1 | 1kbp ladder |
| 2 | Pcon entA1 |
| 3 | Pcon entA2 |
| 4 | Pcon entA3 |
| 5 | 1kbp ladder |
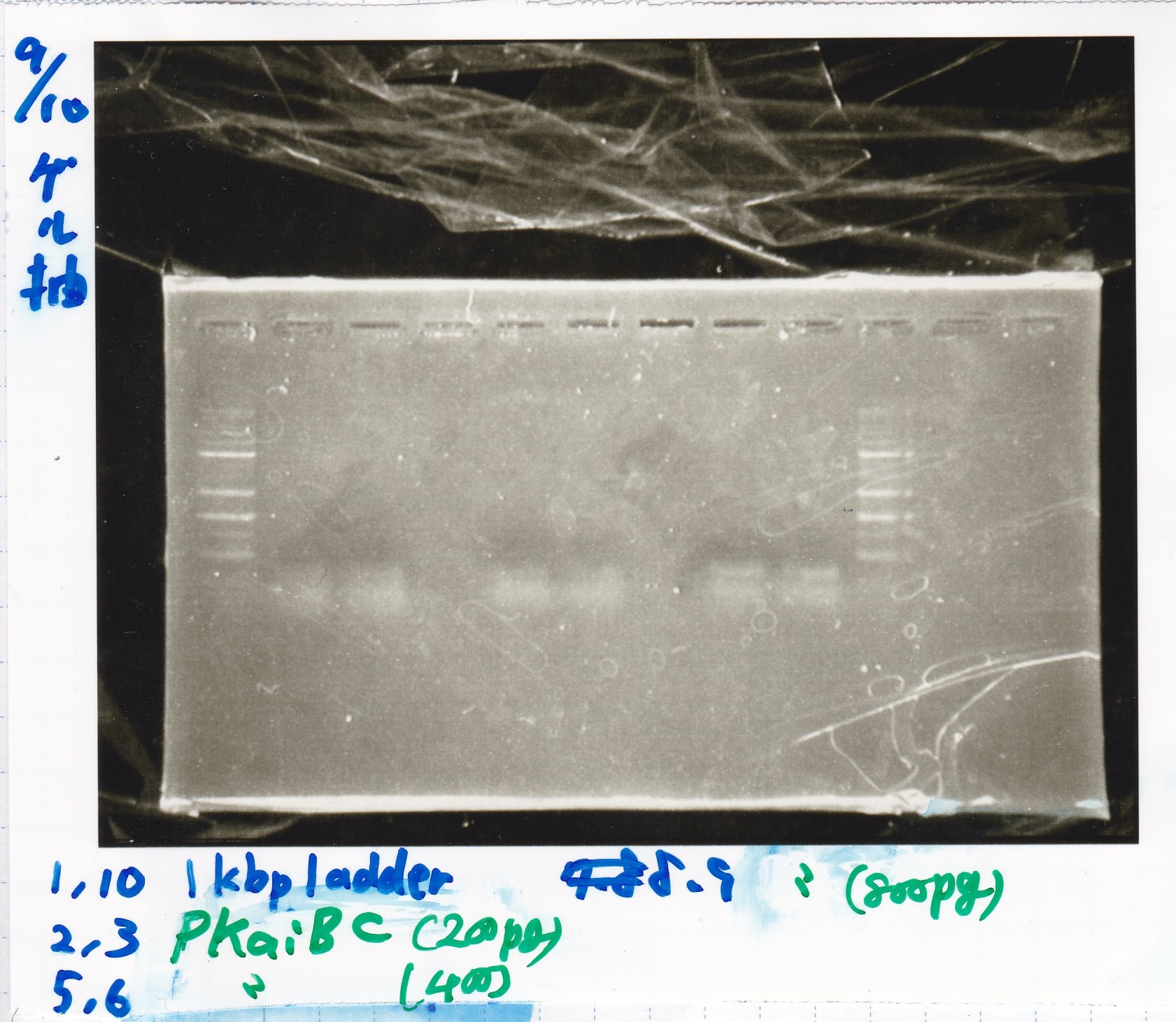
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | -- |
| 2 | KaiA(200pg) | -- |
| 3 | KaiA(200pg) | -- |
| 4 | -- | -- |
| 5 | KaiA(400pg) | -- |
| 6 | KaiA(400pg) | -- |
| 7 | -- | -- |
| 8 | KaiA(800pg | -- |
| 9 | KaiA(800pg) | -- |
| 10 | 1kbp ladder | -- |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | -- |
| 2 | PKaiBC(200pg) | -- |
| 3 | PKaiBC(200pg) | -- |
| 4 | -- | -- |
| 5 | PKaiBC(400pg) | -- |
| 6 | PKaiBC(400pg) | -- |
| 7 | -- | -- |
| 8 | PKaiBC(800pg | -- |
| 9 | PKaiBC(800pg) | -- |
| 10 | 1kbp ladder | -- |
Liquid Culture
| Sample | medium |
|---|---|
| 9/4 J23100①(JM109) | LB(Amp) |
| 9/8 J23100① (entA) | LB(Amp) |
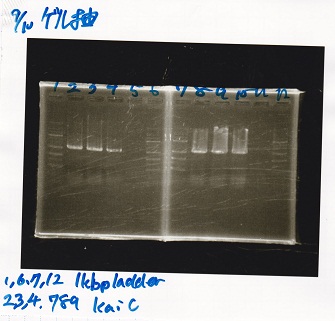
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | -- |
| 2 | KaiC | -- |
| 3 | KaiC | -- |
| 4 | KaiC | -- |
| 5 | -- | -- |
| 6 | 1kbp ladder | -- |
| 7 | 1kbp ladder | -- |
| 8 | KaiC | -- |
| 9 | KaiC | -- |
| 10 | KaiC | -- |
| 11 | -- | -- |
| 12 | 1kbp ladder | -- |
Liquid Culture
| Sample | medium |
|---|---|
| 9/10 Pcon-pt181 antisense-Spinach-DT | Plusgrow |
Colony PCR
| Sample | base pair |
|---|---|
| 9/9 Phad larac-1 RBS-lux1-DT | 2331 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 2min | 30 |
| Sample | base pair |
|---|---|
| 9/9 Pcon-lacZα (psB4KS) | 712 |
| 9/9 Pcon-Spinach-DT(psB4KS) | 605 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 42s | 30 |
| Sample | base pair |
|---|---|
| 9/9 Plac+RBS-lysis3}-DT | 1273 |
| 9/9 Pcon+RBS-lux1-DT | 1079 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 1min12s | 30 |
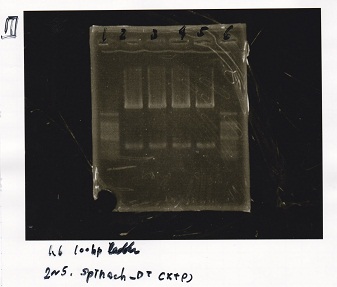
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | 9/9 Pcou-RBS-lacZα-DT(p3B4KS9)-1 | -- | -- |
| 3 | 9/9 Pcou-RBS-lacZα-DT(p3B4KS9)-2 | -- | -- |
| 4 | 9/9 Pcou-Spinach-DT(pB4KS)-1 | -- | -- |
| 5 | 9/9 Pcou-Spinach-DT(pB4KS)-2 | -- | -- |
| 6 | 100bp ladder | -- | -- |
Transformation
| Name | Plate |
|---|---|
| F1 attenuator +p3B1C3 | CP |
| F3n2 attenuator +p3B1C3 | CP |
| F1 antisense +p3B1C3 | CP |
| F6 antisense+p3B1C3 | CP |
| apt12-P +p3B1C3 | CP |
| apt12-1M +p3B1C3 | CP |
| Pcon-luxR+Plux-GFP | Amp |
| Plux+RBS-lysis1-DT | CP |
| Plux+RBS-lysis2-DT | CP |
| Ptet-pT181antisense+Spinach-DT | CP |
| Pcon-pT181 attenuator+apt121R-DT | Amp |
| Pcon-1 apt12-1R-DT | Amp |
| DT+Pcon-pT181 attenuator | CP |
Electrophoresis
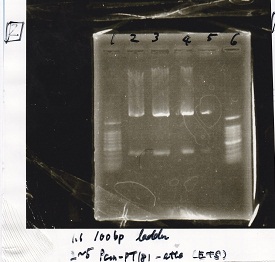
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | Spinach-DT | XbaI | PstI |
| 3 | Spinach-DT | XbaI | PstI |
| 4 | Spinach-DT | XbaI | PstI |
| 5 | Spinach-DT | XbaI | PstI |
| 6 | 100bp ladder | -- | -- |
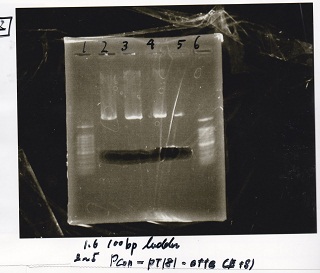
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | Pcon-pT181 attenuator | EcoRI | SpeI |
| 3 | Pcon-pT181 attenuator | EcoRI | SpeI |
| 4 | Pcon-pT181 attenuator | EcoRI | Spei |
| 5 | Pcon-pT181 attenuator | EcoRI | SpeI |
| 6 | 100bp ladder | -- | -- |
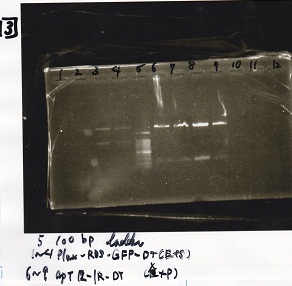
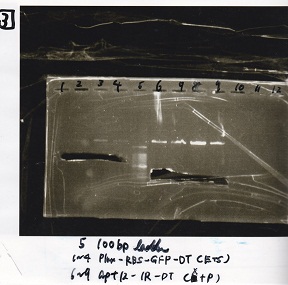
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | Plux-RBS-GFP-DT | EcoRI | SpeI |
| 2 | Plux-RBS-GFP-DT | EcoRI | SpeI |
| 3 | Plux-RBS-GFP-DT | EcoRI | SpeI |
| 4 | Plux-RBS-GFP-DT | EcoRI | SpeI |
| 5 | 100bp ladder | -- | -- |
| 6 | apt12-1R-DT | XbaI | PstI |
| 7 | apt12-1R-DT | XbaI | PstI |
| 8 | apt12-1R-DT | XbaI | PstI |
| 9 | apt12-1R-DT | XbaI | PstI |
 "
"