Template:Kyoto/Notebook/Sep 5
From 2013.igem.org
(Difference between revisions)
(→Electrophoresis) |
(→Restriction Enzyme Digestion) |
||
| (44 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
==Sep 5== | ==Sep 5== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
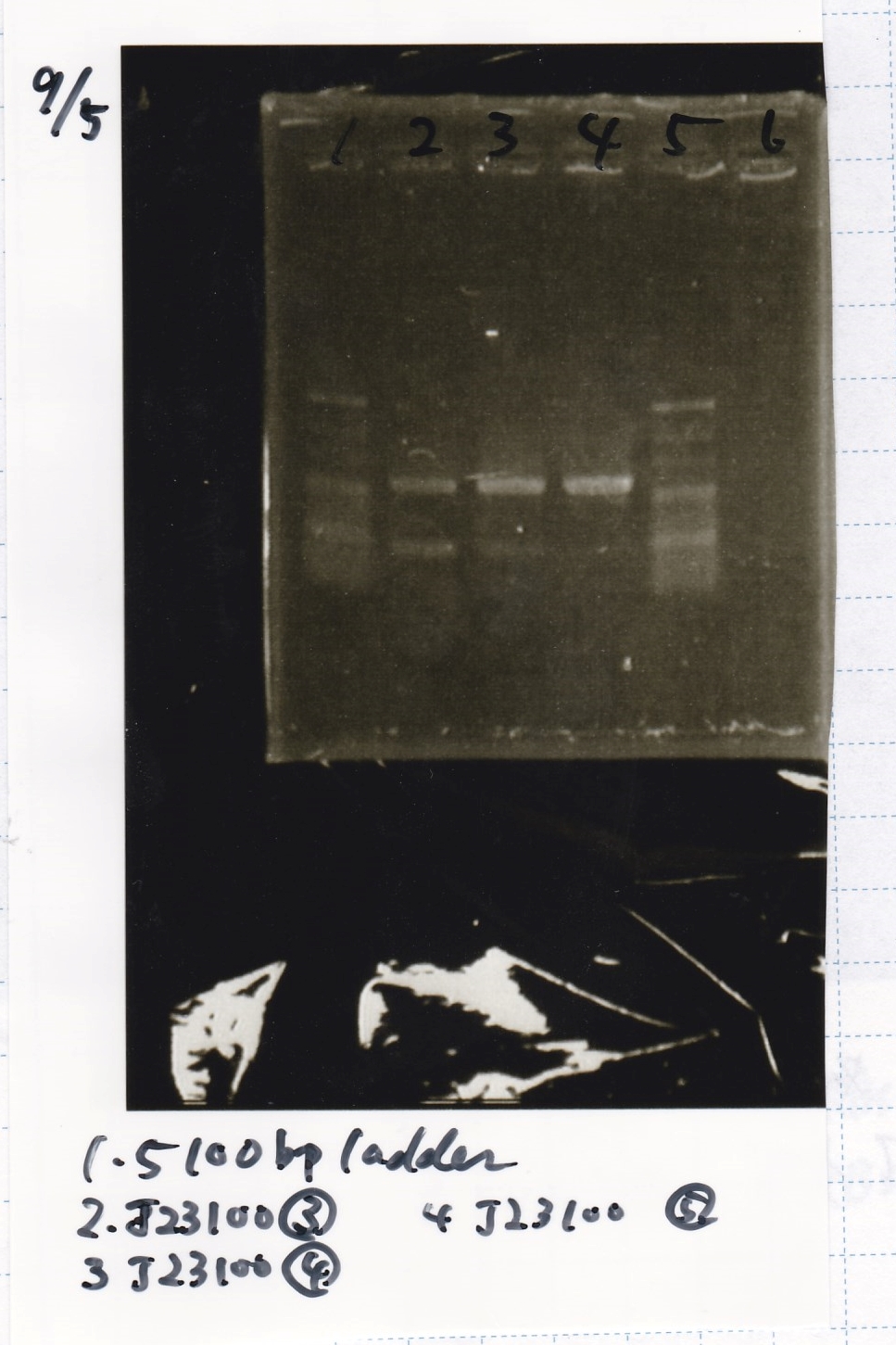
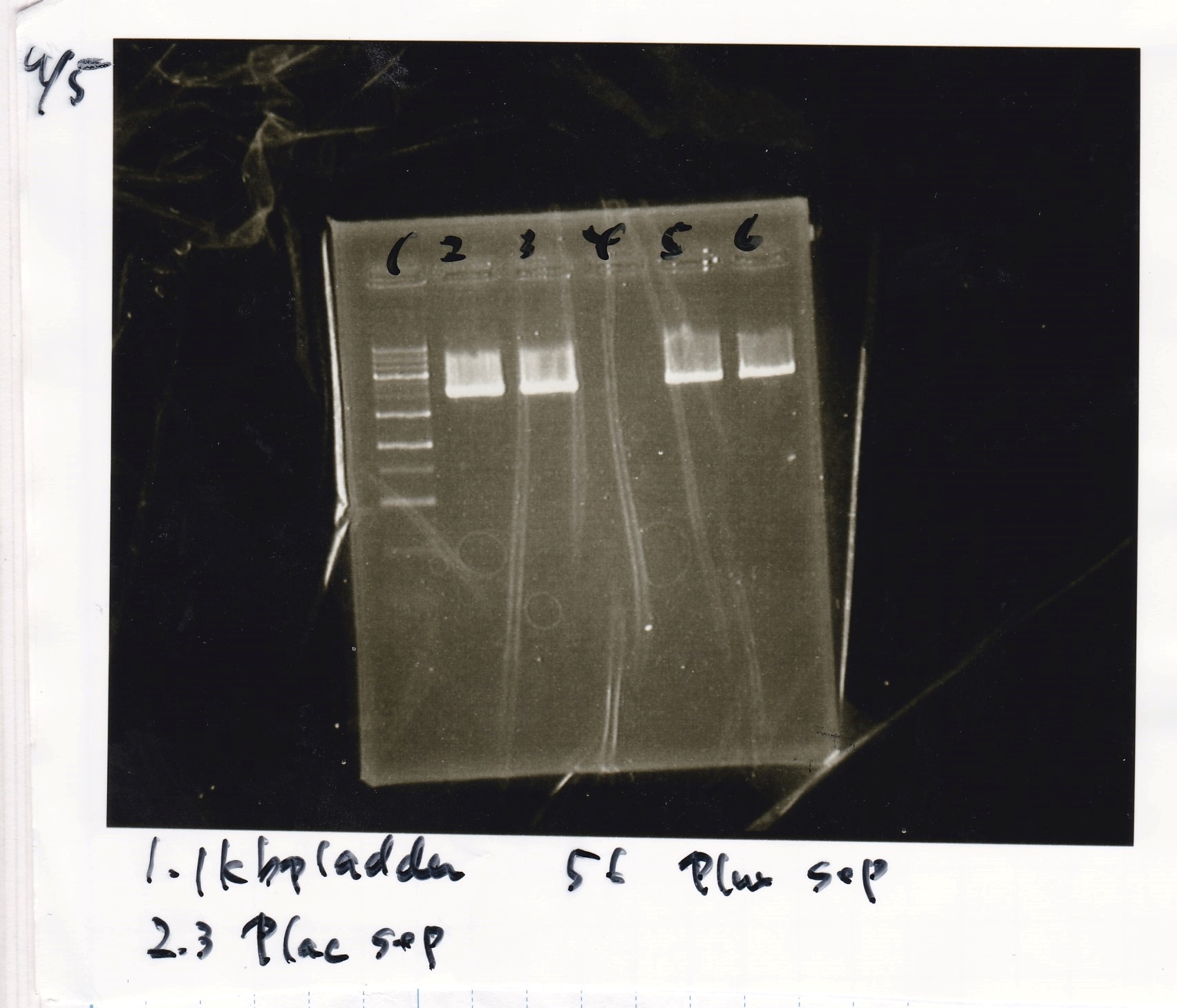
===Electrophoresis=== | ===Electrophoresis=== | ||
<div class="experiment"> | <div class="experiment"> | ||
| Line 37: | Line 16: | ||
|5||100bp ladder||--||-- | |5||100bp ladder||--||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_Sep5_electrophoresis_N1.jpg]]<br> |
</div> | </div> | ||
| Line 75: | Line 54: | ||
|} | |} | ||
incubate 16 °C 1 hour | incubate 16 °C 1 hour | ||
| + | </div> | ||
| + | |||
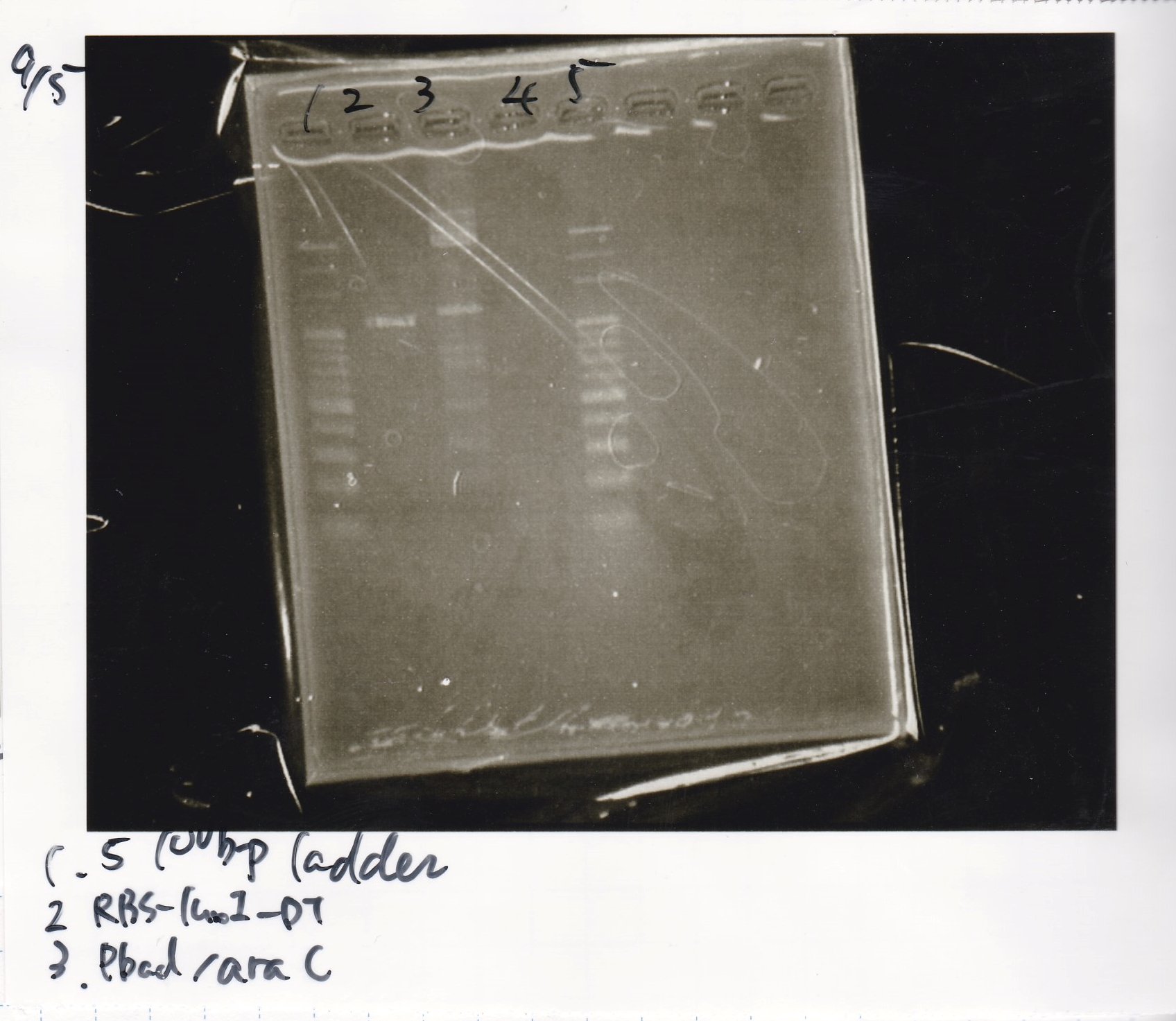
| + | ===Electrophoresis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Tatsui</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||100bp ladder | ||
| + | |- | ||
| + | |2||RBS-luxI-DT | ||
| + | |- | ||
| + | |3||Pbad/araC | ||
| + | |- | ||
| + | |4||NC | ||
| + | |- | ||
| + | |5||100bp ladder | ||
| + | |} | ||
| + | [[File:igku_Sep5_electrophorsis_N2_1.jpg]]<br> | ||
| + | [[File:igku_Sep5_electrophorsis_N2_2.jpg]]<br> | ||
</div> | </div> | ||
| Line 89: | Line 88: | ||
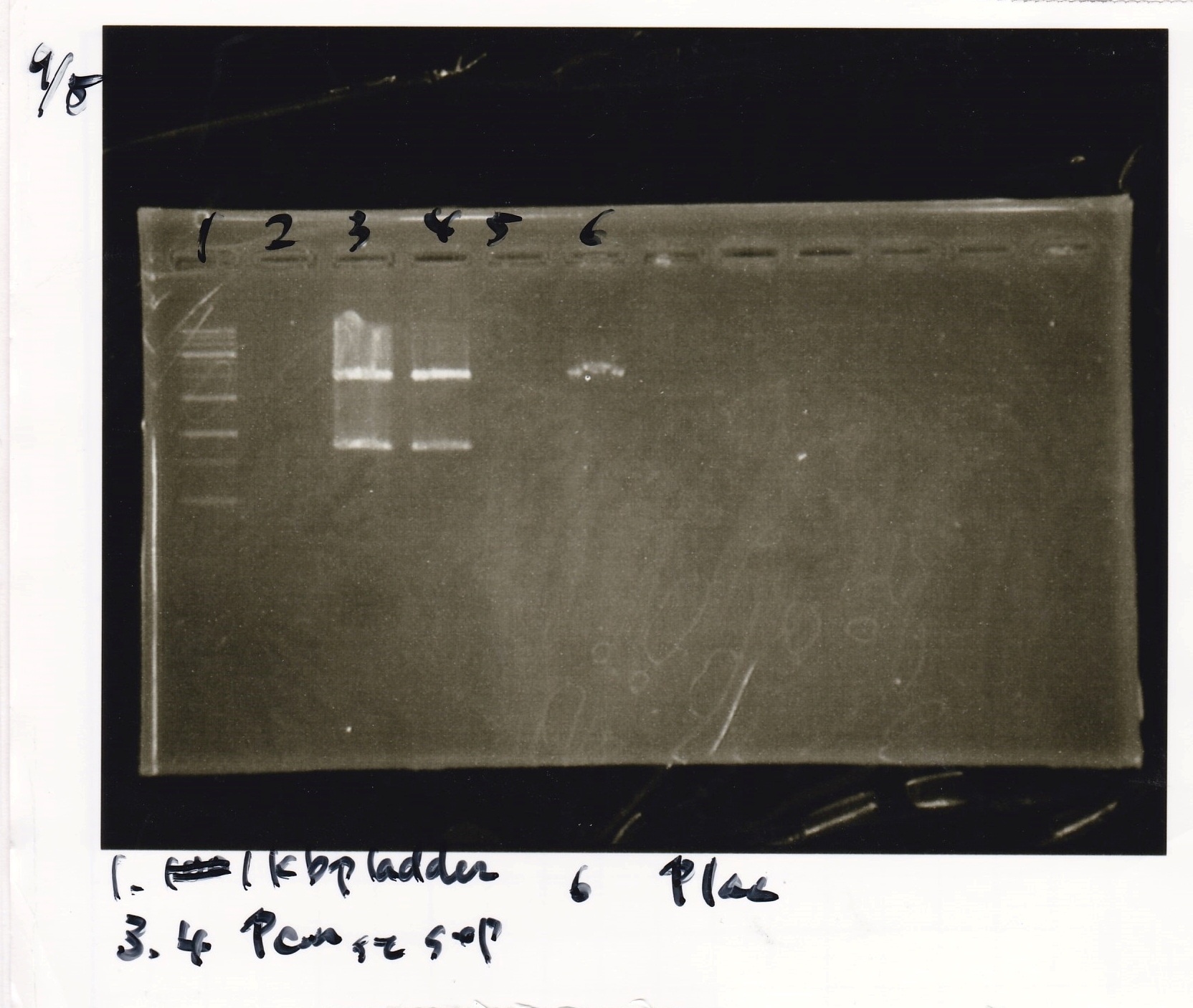
|3||RBS-luxI-DT|| | |3||RBS-luxI-DT|| | ||
|- | |- | ||
| - | |4|| | + | |4|||| |
|- | |- | ||
|5||Pbad/araC|| | |5||Pbad/araC|| | ||
| Line 95: | Line 94: | ||
|6||Pbad/araC|| | |6||Pbad/araC|| | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Sep5 Gel Extraction(N3) 1.jpg]]<br> |
| - | [[File: | + | [[File:Igku Sep5 Gel Extraction(N3) 2.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Name||concentration[µg/mL]||260/280||260/230 | !Name||concentration[µg/mL]||260/280||260/230 | ||
| Line 111: | Line 110: | ||
!Sample||base pair | !Sample||base pair | ||
|- | |- | ||
| - | |9/4 | + | |9/4 aptamer 12_1R(pSB1C3)-1||384 |
|- | |- | ||
| - | |9/4 | + | |9/4 aptamer 12_1R(pSB1C3)-2||384 |
|- | |- | ||
|9/4 pT181 antisense(pSB1C3)-1||415 | |9/4 pT181 antisense(pSB1C3)-1||415 | ||
| Line 134: | Line 133: | ||
!Sample||base pair | !Sample||base pair | ||
|- | |- | ||
| - | |9/4 | + | |9/4 aptamer 12_1R-DT-1||521 |
|- | |- | ||
| - | |9/4 | + | |9/4 aptamer 12_1R-DT-2||521 |
|- | |- | ||
| - | |9/4 | + | |9/4 aptamer 12_1R-DT-3||521 |
|- | |- | ||
| - | |9/4 | + | |9/4 aptamer 12_1R-DT-4||521 |
|- | |- | ||
|9/4 pT181 attenuator(pSB1C3)||601 | |9/4 pT181 attenuator(pSB1C3)||601 | ||
| Line 181: | Line 180: | ||
|5min||30s||30s||72s||30cycles | |5min||30s||30s||72s||30cycles | ||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
</div> | </div> | ||
| Line 281: | Line 209: | ||
|9/5 Ptet+pT181 antisense||2||20||22||-- | |9/5 Ptet+pT181 antisense||2||20||22||-- | ||
|- | |- | ||
| - | |9/5 Plux+pT181 | + | |9/5 Plux+pT181 attenuator||2||20||22||-- |
|- | |- | ||
| - | |9/5 Ptet+pT181 | + | |9/5 Ptet+pT181 attenuator||2||20||22||-- |
|- | |- | ||
| - | |9/5 Ptet+pT181 | + | |9/5 Ptet+pT181 attenuator||2||20||22||-- |
|- | |- | ||
|9/5 Pconst+RBS-tetR+DT||2||20||22||-- | |9/5 Pconst+RBS-tetR+DT||2||20||22||-- | ||
| Line 298: | Line 226: | ||
<span class="author">No name</span> | <span class="author">No name</span> | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||9/5 Pbad/araC|| | + | ! ||9/5 Pbad/araC||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts||4µL|| 0µL|| 1µL|| 0µL|| 1µL|| 3µL|| 3µL|| 18µL|| 30µL | |2 cuts||4µL|| 0µL|| 1µL|| 0µL|| 1µL|| 3µL|| 3µL|| 18µL|| 30µL | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||9/5 RBS-luxI-DT|| | + | ! ||9/5 RBS-luxI-DT||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts||4µL|| 0µL|| 0µL|| 1µL|| 1µL|| 3µL|| 3µL||18µL|| 30µL | |2 cuts||4µL|| 0µL|| 0µL|| 1µL|| 1µL|| 3µL|| 3µL||18µL|| 30µL | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||8/28 Plux|| | + | ! ||8/28 Plux||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts|| 12µL|| 0µL|| 1µL|| 0µL|| 1µL|| 3µL|| 3µL||10µL||30µL | |2 cuts|| 12µL|| 0µL|| 1µL|| 0µL|| 1µL|| 3µL|| 3µL||10µL||30µL | ||
| Line 315: | Line 243: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||9/5 Pconst(J23100)|| | + | ! ||9/5 Pconst(J23100)||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts||5.7µL||0µL||1µL||0µL||1µL||3µL||3µL||16.3µL||30µL | |2 cuts||5.7µL||0µL||1µL||0µL||1µL||3µL||3µL||16.3µL||30µL | ||
| Line 322: | Line 250: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||9/5 Plac|| | + | ! ||9/5 Plac||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts||6.0µL||0µL||1µL||0µL||1µL||3µL||3µL||16µL||30µL | |2 cuts||6.0µL||0µL||1µL||0µL||1µL||3µL||3µL||16µL||30µL | ||
| Line 329: | Line 257: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||8/31 Plux-RBS-GFP-DT-1|| | + | ! ||8/31 Plux-RBS-GFP-DT-1||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts||9.4µL||1µL||1µL||0µL||0µL||3µL||3µL||12.6µL||30µL | |2 cuts||9.4µL||1µL||1µL||0µL||0µL||3µL||3µL||12.6µL||30µL | ||
| Line 336: | Line 264: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! ||8/20 Pconst-RBS-luxR-DT|| | + | ! ||8/20 Pconst-RBS-luxR-DT||EcoRI||SpeI||XbaI||PstI||BSA||Buffer||MilliQ||total |
|- | |- | ||
|2 cuts||4.5µL||1µL||0µL||1µL||0µL||3µL||3µL||13.5µL||30µL | |2 cuts||4.5µL||1µL||0µL||1µL||0µL||3µL||3µL||13.5µL||30µL | ||
| Line 355: | Line 283: | ||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">No name</span> | <span class="author">No name</span> | ||
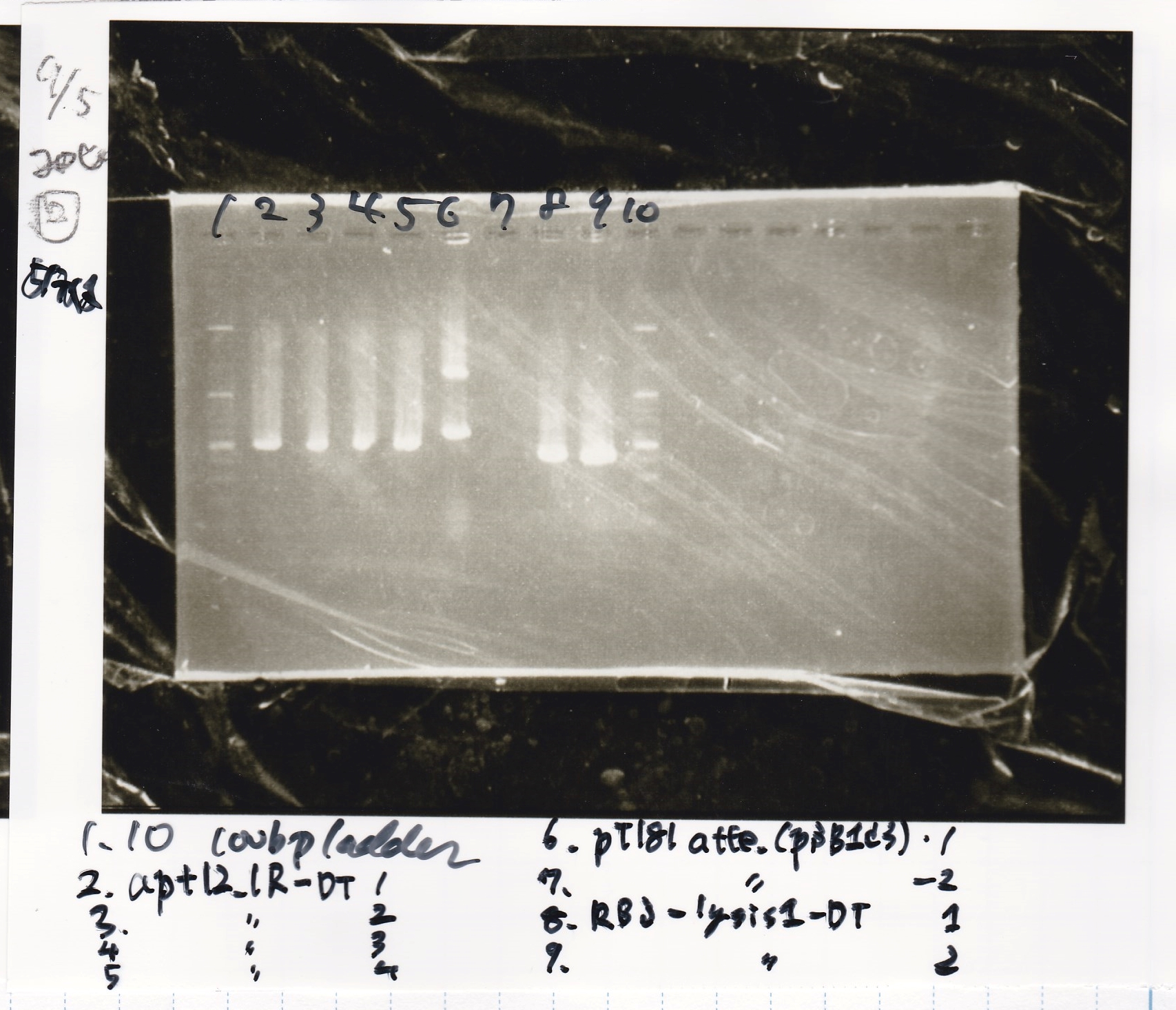
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||100bp ladder||--||-- | ||
| + | |- | ||
| + | |2||aptamer 12_1R (pSB1C3)-1||--||-- | ||
| + | |- | ||
| + | |3||aptamer 12_1R (pSB1C3)-2||--||-- | ||
| + | |- | ||
| + | |4||pT181 antisense(pSB1C3)-1||--||-- | ||
| + | |- | ||
| + | |5||pT181 antisense(pSB1C3)-2||--||-- | ||
| + | |- | ||
| + | |6||pT181 antisense(pSB1C3)-3||--||-- | ||
| + | |- | ||
| + | |7||pT181 antisense(pSB1C3)-4||--||-- | ||
| + | |- | ||
| + | |8||100bp ladder||--||-- | ||
| + | |} | ||
| + | [[File:Igku Sep5 Electrophoresis(N4).jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||100bp ladder||--||-- | ||
| + | |- | ||
| + | |2||aptamer 12_1R-DT-1||--||-- | ||
| + | |- | ||
| + | |3||aptamer 12_1R-DT-2||--||-- | ||
| + | |- | ||
| + | |4||aptamer 12_1R-DT-3||--||-- | ||
| + | |- | ||
| + | |5||aptamer 12_1R-DT-4||--||-- | ||
| + | |- | ||
| + | |6||pT181 attenuator(pSB1C3)-1||--||-- | ||
| + | |- | ||
| + | |7||pT181 attenuator(pSB1C3)-2||--||-- | ||
| + | |- | ||
| + | |8||RBS-lysis1-DT-1||--||-- | ||
| + | |- | ||
| + | |9||RBS-lysis1-DT-2||--||-- | ||
| + | |- | ||
| + | |10||100bp ladder||--||-- | ||
| + | |} | ||
| + | [[File:Igku Sep5 Electrophoresis(N6) 2.jpg]]<br> | ||
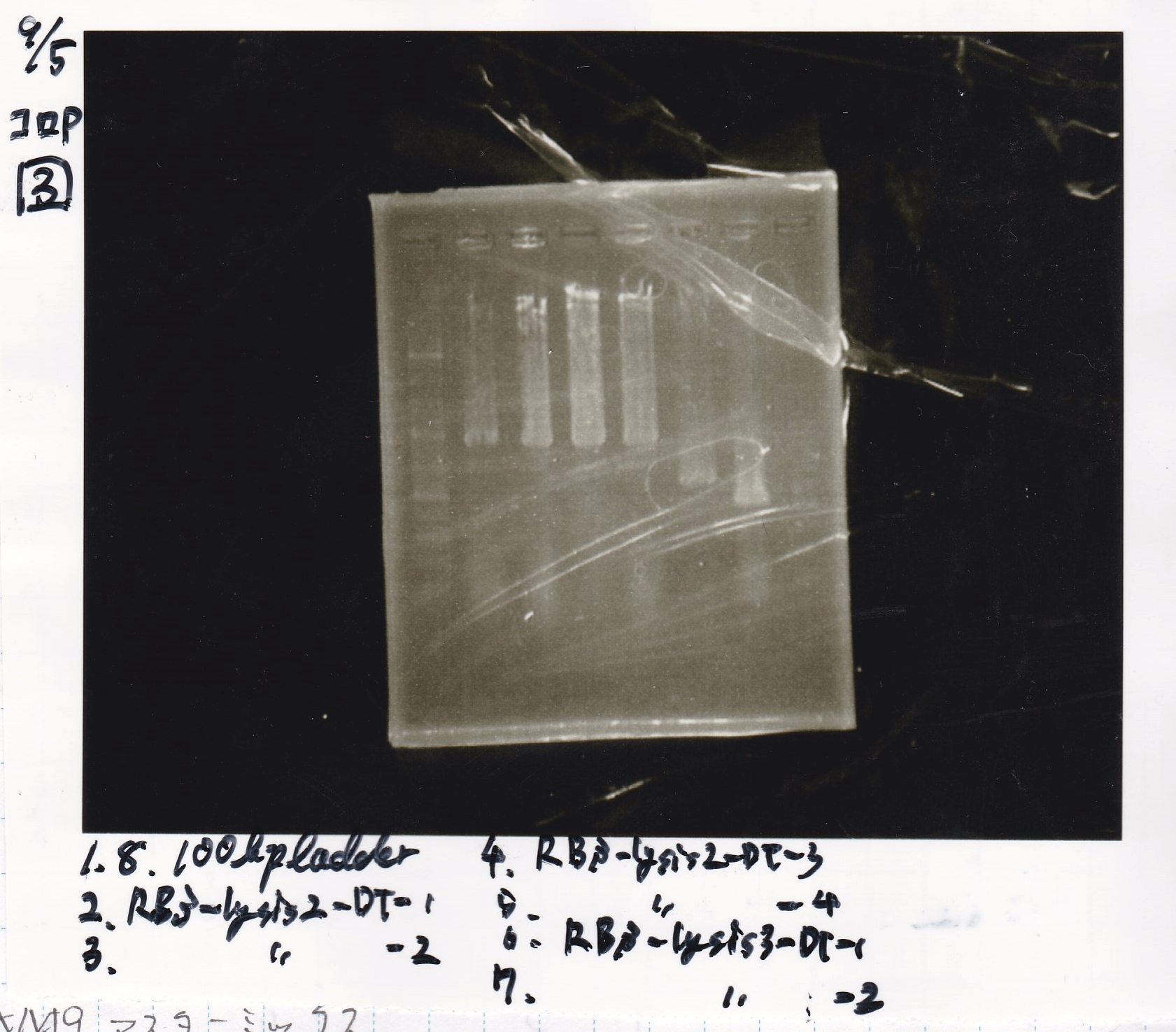
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||100bp ladder||--||-- | ||
| + | |- | ||
| + | |2||RBS-lysis2-DT-1||--||-- | ||
| + | |- | ||
| + | |3||RBS-lysis2-DT-2||--||-- | ||
| + | |- | ||
| + | |4||RBS-lysis2-DT-3||--||-- | ||
| + | |- | ||
| + | |5||RBS-lysis2-DT-4||--||-- | ||
| + | |- | ||
| + | |6||RBS-lysis3-DT-1||--||-- | ||
| + | |- | ||
| + | |7||RBS-lysis3-DT-2||--||-- | ||
| + | |- | ||
| + | |8||100bp ladder||--||-- | ||
| + | |} | ||
| + | [[File:Igku Sep5 Electrophoresis(N5).jpg]]<br> | ||
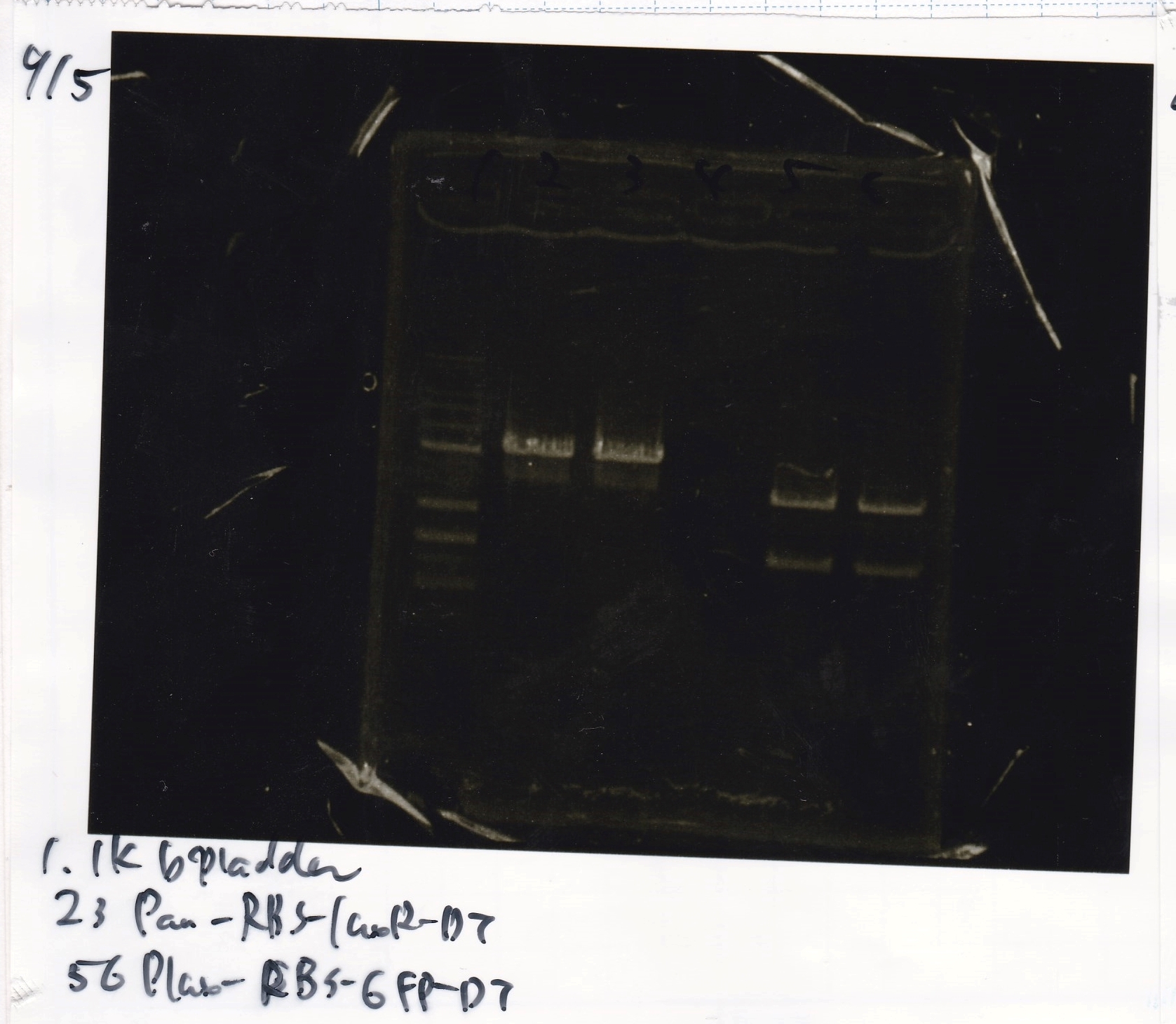
{| class="wikitable" | {| class="wikitable" | ||
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
| Line 362: | Line 354: | ||
|2||Pconst||SpeI||PstI | |2||Pconst||SpeI||PstI | ||
|- | |- | ||
| - | |3||Pconst||--||-- | + | |3||Pconst NC||--||-- |
|- | |- | ||
|4||Plac||SpeI||PstI | |4||Plac||SpeI||PstI | ||
|- | |- | ||
| - | |5||Plac||--||-- | + | |5||Plac NC||--||-- |
|- | |- | ||
|6||Plux||SpeI||PstI | |6||Plux||SpeI||PstI | ||
|- | |- | ||
| - | |7||Plux||--||-- | + | |7||Plux NC||--||-- |
|- | |- | ||
|8||Pconst-RBS-luxR-DT||EcoRI||XbaI | |8||Pconst-RBS-luxR-DT||EcoRI||XbaI | ||
|- | |- | ||
| - | |9||Pconst-RBS-luxR-DT||--||-- | + | |9||Pconst-RBS-luxR-DT NC||--||-- |
|- | |- | ||
|10||Plux-RBS-GFP-DT||EcoRI||SpeI | |10||Plux-RBS-GFP-DT||EcoRI||SpeI | ||
|- | |- | ||
| - | |11||Plux-RBS-GFP-DT||--||-- | + | |11||Plux-RBS-GFP-DT NC||--||-- |
|- | |- | ||
|12||1kbp ladder||--||-- | |12||1kbp ladder||--||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Sep5 Electrophoresis(N6) 3.jpg]]<br> |
</div> | </div> | ||
| - | === | + | ===5x M9 Medium (+EDTA)=== |
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">Hirano</span> | <span class="author">Hirano</span> | ||
| Line 399: | Line 391: | ||
|NH4Cl||10mg | |NH4Cl||10mg | ||
|- | |- | ||
| - | |Fe( | + | |Fe(III)-EDTA||1263.27mg |
| + | |- | ||
| + | |MilliQ||up to 10 mL | ||
|} | |} | ||
| + | *autoclave at 121 °C for 20 min | ||
</div> | </div> | ||
| - | |||
===Gel Extraction=== | ===Gel Extraction=== | ||
| Line 416: | Line 410: | ||
|3||Pconst||SpeI & PstI | |3||Pconst||SpeI & PstI | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_Sep5_gel_extraction_N7_1.jpg]]<br> |
| - | [[File: | + | [[File:igku_Sep5_gel_extraction_N7_2.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Name||concentration[µg/mL]||260/280||260/230 | !Name||concentration[µg/mL]||260/280||260/230 | ||
| Line 436: | Line 430: | ||
|6||Plux||SpeI & PstI | |6||Plux||SpeI & PstI | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_Sep5_gel_extraction_N7_3.jpg]]<br> |
| - | [[File: | + | [[File:igku_Sep5_gel_extraction_N7_4.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Name||concentration[µg/mL]||260/280||260/230 | !Name||concentration[µg/mL]||260/280||260/230 | ||
| Line 458: | Line 452: | ||
|6||Plux-RBS-GFP-DT||EcoRI & SpeI | |6||Plux-RBS-GFP-DT||EcoRI & SpeI | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_Sep5_gel_extraction_N7_5.jpg]]<br> |
| - | [[File: | + | [[File:igku_Sep5_gel_extraction_N7_6.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
!Name||concentration[µg/mL]||260/280||260/230 | !Name||concentration[µg/mL]||260/280||260/230 | ||
| Line 467: | Line 461: | ||
|Plux-RBS-GFP-DT (EcoRI & SpeI)||21.8||1.87||0.98 | |Plux-RBS-GFP-DT (EcoRI & SpeI)||21.8||1.87||0.98 | ||
|} | |} | ||
| + | |||
| + | ===DNA Purification=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {|class="wikitable" | ||
| + | !DNA||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |9/5 RBS-luxI-DT||7.6||1.56||-1.42 | ||
| + | |- | ||
| + | |9/5 Pbad/araC||7.5||1.54||3.84 | ||
| + | |} | ||
| + | </div> | ||
===Miniprep=== | ===Miniprep=== | ||
| Line 500: | Line 506: | ||
|94°C||94°C||55°C||68°C||-- | |94°C||94°C||55°C||68°C||-- | ||
|- | |- | ||
| - | | | + | |5min||30s||30s||1min 25s||30cycles |
|} | |} | ||
</div> | </div> | ||
| - | === | + | ===Liquid Culture=== |
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">Hirano</span> |
{| class="wikitable" | {| class="wikitable" | ||
| - | ! | + | !Sample||medium |
|- | |- | ||
| - | | | + | |9/4 aptamer 12_1R-DT-1||Plusgrow medium (+CP) |
|- | |- | ||
| - | | | + | |9/4 pT181 attenuator(pSB1C3)-1||Plusgrow medium (+CP) |
|- | |- | ||
| - | | | + | |9/4 RBS-lysis2-DT-1||Plusgrow medium (+CP) |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|- | |- | ||
| - | | | + | |9/4 Ptrc-KaiC -1||Plusgrow medium (+Amp) |
|- | |- | ||
| + | |9/4 pSB4K5 -1||Plusgrow medium (+Kan) | ||
|} | |} | ||
</div> | </div> | ||
Latest revision as of 14:20, 27 September 2013
Sep 5
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | J23100-3 | -- | -- |
| 3 | J23100-4 | -- | -- |
| 4 | J23100-5 | -- | -- |
| 5 | 100bp ladder | -- | -- |
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 8/3 J23100-3 | 349.1 | 1.69 | 1.851 |
| 8/10 Plac-2 | 331.3 | 1.84 | 1.42 |
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | Pconst | 13.2 | spinach-DT | 2.1 | 3.5 |
| experiment | Plac | 15.3 | spinach-DT | 2.1 | 3.5 |
| experiment | Pconst | 13.2 | pT181 antisense (XbaI & PstI) | 6.6 | 3.5 |
| experiment | Plac | 15.3 | pT181 antisense (XbaI & PstI) | 6.6 | 3.5 |
| experiment | Ptet | 30.1 | pT181 antisense (XbaI & PstI) | 6.6 | 3.5 |
| experiment | Pconst | 13.2 | pT181 attenuator | 11.7 | 3.5 |
| experiment | Plac | 15.3 | pT181 attenuator | 11.7 | 3.5 |
| experiment | Ptet | 30.1 | RBS-lacZα-DT | 3.0 | 3.5 |
incubate 16 °C 1 hour
Electrophoresis
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | |
| 2 | RBS-luxI-DT | |
| 3 | RBS-luxI-DT | |
| 4 | ||
| 5 | Pbad/araC | |
| 6 | Pbad/araC |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| RBS-luxI-DT | 23.2 | 0.83 | 7.89 |
| Pbad/araC | 51.8 | 1.20 | 0.11 |
Colony PCR
| Sample | base pair |
|---|---|
| 9/4 aptamer 12_1R(pSB1C3)-1 | 384 |
| 9/4 aptamer 12_1R(pSB1C3)-2 | 384 |
| 9/4 pT181 antisense(pSB1C3)-1 | 415 |
| 9/4 pT181 antisense(pSB1C3)-2 | 415 |
| 9/4 pT181 antisense(pSB1C3)-3 | 415 |
| 9/4 pT181 antisense(pSB1C3)-4 | 415 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 30s | 30cycles |
| Sample | base pair |
|---|---|
| 9/4 aptamer 12_1R-DT-1 | 521 |
| 9/4 aptamer 12_1R-DT-2 | 521 |
| 9/4 aptamer 12_1R-DT-3 | 521 |
| 9/4 aptamer 12_1R-DT-4 | 521 |
| 9/4 pT181 attenuator(pSB1C3) | 601 |
| 9/4 pT181 attenuator(pSB1C3) | 601 |
| 9/4 RBS-lysis1-DT | 613 |
| 9/4 RBS-lysis1-DT | 613 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 36s | 30cycles |
| Sample | base pair |
|---|---|
| 9/4 RBS-lysis2-DT-1 | 985 |
| 9/4 RBS-lysis2-DT-2 | 985 |
| 9/4 RBS-lysis2-DT-3 | 985 |
| 9/4 RBS-lysis2-DT-4 | 985 |
| 9/4 RBS-lysis3-DT-1 | 1210 |
| 9/4 RBS-lysis3-DT-2 | 1210 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 72s | 30cycles |
Liquid Culture
| Sample | medium |
|---|---|
| 8/9 J23100-5 | Plusgrow medium(+Amp) |
37°C
Transformation
| Name | Sample(µL) | Competent Cells(µL) | Total(µL) | Plate |
|---|---|---|---|---|
| 9/5 Pconst+spinach-DT | 2 | 20 | 22 | -- |
| 9/5 Plac+spinach-DT | 2 | 20 | 22 | -- |
| 9/5 Pconst+pT181 antisense | 2 | 20 | 22 | -- |
| 9/5 Plac+pT181 antisense | 2 | 20 | 22 | -- |
| 9/5 Ptet+pT181 antisense | 2 | 20 | 22 | -- |
| 9/5 Plux+pT181 attenuator | 2 | 20 | 22 | -- |
| 9/5 Ptet+pT181 attenuator | 2 | 20 | 22 | -- |
| 9/5 Ptet+pT181 attenuator | 2 | 20 | 22 | -- |
| 9/5 Pconst+RBS-tetR+DT | 2 | 20 | 22 | -- |
| 9/16(2012) T7-His-FT | 2 | 20 | 22 | -- |
| 9/16(2012) pBr322 | 2 | 20 | 22 | -- |
Restriction Enzyme Digestion
| 9/5 Pbad/araC | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 4µL | 0µL | 1µL | 0µL | 1µL | 3µL | 3µL | 18µL | 30µL |
| 9/5 RBS-luxI-DT | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 4µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 18µL | 30µL |
| 8/28 Plux | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 12µL | 0µL | 1µL | 0µL | 1µL | 3µL | 3µL | 10µL | 30µL |
| NC | 0.6µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.4µL | 10µL |
| 9/5 Pconst(J23100) | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 5.7µL | 0µL | 1µL | 0µL | 1µL | 3µL | 3µL | 16.3µL | 30µL |
| NC | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL |
| 9/5 Plac | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 6.0µL | 0µL | 1µL | 0µL | 1µL | 3µL | 3µL | 16µL | 30µL |
| NC | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL |
| 8/31 Plux-RBS-GFP-DT-1 | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 9.4µL | 1µL | 1µL | 0µL | 0µL | 3µL | 3µL | 12.6µL | 30µL |
| NC | 0.5µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL |
| 8/20 Pconst-RBS-luxR-DT | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 4.5µL | 1µL | 0µL | 1µL | 0µL | 3µL | 3µL | 13.5µL | 30µL |
| NC | 0.2µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.8µL | 10µL |
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 9/5 RBS-luxI-DT | 7.6 | 1.56 | -1.42 |
| 9/5 Pbad/araC | 7.5 | 1.54 | 3.84 |
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | aptamer 12_1R (pSB1C3)-1 | -- | -- |
| 3 | aptamer 12_1R (pSB1C3)-2 | -- | -- |
| 4 | pT181 antisense(pSB1C3)-1 | -- | -- |
| 5 | pT181 antisense(pSB1C3)-2 | -- | -- |
| 6 | pT181 antisense(pSB1C3)-3 | -- | -- |
| 7 | pT181 antisense(pSB1C3)-4 | -- | -- |
| 8 | 100bp ladder | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | aptamer 12_1R-DT-1 | -- | -- |
| 3 | aptamer 12_1R-DT-2 | -- | -- |
| 4 | aptamer 12_1R-DT-3 | -- | -- |
| 5 | aptamer 12_1R-DT-4 | -- | -- |
| 6 | pT181 attenuator(pSB1C3)-1 | -- | -- |
| 7 | pT181 attenuator(pSB1C3)-2 | -- | -- |
| 8 | RBS-lysis1-DT-1 | -- | -- |
| 9 | RBS-lysis1-DT-2 | -- | -- |
| 10 | 100bp ladder | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | RBS-lysis2-DT-1 | -- | -- |
| 3 | RBS-lysis2-DT-2 | -- | -- |
| 4 | RBS-lysis2-DT-3 | -- | -- |
| 5 | RBS-lysis2-DT-4 | -- | -- |
| 6 | RBS-lysis3-DT-1 | -- | -- |
| 7 | RBS-lysis3-DT-2 | -- | -- |
| 8 | 100bp ladder | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 1kbp ladder | -- | -- |
| 2 | Pconst | SpeI | PstI |
| 3 | Pconst NC | -- | -- |
| 4 | Plac | SpeI | PstI |
| 5 | Plac NC | -- | -- |
| 6 | Plux | SpeI | PstI |
| 7 | Plux NC | -- | -- |
| 8 | Pconst-RBS-luxR-DT | EcoRI | XbaI |
| 9 | Pconst-RBS-luxR-DT NC | -- | -- |
| 10 | Plux-RBS-GFP-DT | EcoRI | SpeI |
| 11 | Plux-RBS-GFP-DT NC | -- | -- |
| 12 | 1kbp ladder | -- | -- |
5x M9 Medium (+EDTA)
| volume | 10ml |
|---|---|
| Na2HPO4 | 60mg |
| KH2PO4 | 30mg |
| NaCl | 5mg |
| NH4Cl | 10mg |
| Fe(III)-EDTA | 1263.27mg |
| MilliQ | up to 10 mL |
- autoclave at 121 °C for 20 min
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | |
| 2 | Pconst | SpeI & PstI |
| 3 | Pconst | SpeI & PstI |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Pconst | 5.3 | 1.98 | 0.06 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | |
| 2 | Plac | SpeI & PstI |
| 3 | Plac | SpeI & PstI |
| 5 | Plux | SpeI & PstI |
| 6 | Plux | SpeI & PstI |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Plac (SpeI & PstI) | 5.2 | 1.82 | 0.36 |
| Plux(SpeI & PstI) | 8.0 | 1.87 | 0.25 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | |
| 2 | Pconst-RBS-luxR-DT | EcoRI & XbaI |
| 3 | Pconst-RBS-luxR-DT | EcoRI & XbaI |
| 5 | Plux-RBS-GFP-DT | EcoRI & SpeI |
| 6 | Plux-RBS-GFP-DT | EcoRI & SpeI |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Pconst-RBS-luxR-DT(EcoRI & XbaI) | 30.6 | 1.84 | 1.16 |
| Plux-RBS-GFP-DT (EcoRI & SpeI) | 21.8 | 1.87 | 0.98 |
DNA Purification
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 9/5 RBS-luxI-DT | 7.6 | 1.56 | -1.42 |
| 9/5 Pbad/araC | 7.5 | 1.54 | 3.84 |
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 8/9 J23100-5 | 243.0 | 1.90 | 1.81 |
Colony PCR
| Sample | base pair |
|---|---|
| 9/4 RBS-lysis3-DT-3 | 1210 |
| 9/4 RBS-lysis3-DT-4 | 1210 |
| 9/4 RBS-lysis3-DT-5 | 1210 |
| 9/4 RBS-lysis1-DT-3 | 613 |
| 9/4 Ptrc KaiC-1 | -- |
| 9/4 pSB4K5 | 1370 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 1min 25s | 30cycles |
Liquid Culture
| Sample | medium |
|---|---|
| 9/4 aptamer 12_1R-DT-1 | Plusgrow medium (+CP) |
| 9/4 pT181 attenuator(pSB1C3)-1 | Plusgrow medium (+CP) |
| 9/4 RBS-lysis2-DT-1 | Plusgrow medium (+CP) |
| 9/4 Ptrc-KaiC -1 | Plusgrow medium (+Amp) |
| 9/4 pSB4K5 -1 | Plusgrow medium (+Kan) |
 "
"