Template:Kyoto/Notebook/Sep 3
From 2013.igem.org
(Difference between revisions)
(→Electrophoresis) |
(→PCR) |
||
| (50 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
==Sep 3== | ==Sep 3== | ||
| + | |||
===Electrophoresis=== | ===Electrophoresis=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">Kojima</span> |
{| class="wikitable" | {| class="wikitable" | ||
!Lane||Sample | !Lane||Sample | ||
| Line 10: | Line 10: | ||
|1||100bp ladder | |1||100bp ladder | ||
|- | |- | ||
| - | |2||pT181 | + | |2||pT181 attenuator(2)-DT2(738) |
|- | |- | ||
|3||Ptet-RBS-lacZα-DT-2(756) | |3||Ptet-RBS-lacZα-DT-2(756) | ||
| Line 29: | Line 29: | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku Sep3 Electrophoresis(N1).jpg]]<br> |
</div> | </div> | ||
| - | |||
===Miniprep=== | ===Miniprep=== | ||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">Tatsui</span> |
{|class="wikitable" | {|class="wikitable" | ||
!DNA||concentration[µg/mL]||260/280||260/230 | !DNA||concentration[µg/mL]||260/280||260/230 | ||
| Line 41: | Line 40: | ||
|9/2 14:00 pT181 attenuator||418.4||1.82||1.80 | |9/2 14:00 pT181 attenuator||418.4||1.82||1.80 | ||
|- | |- | ||
| - | |9/2 14:00 pT181 antisense||362.2||1. | + | |9/2 14:00 pT181 antisense||362.2||1.92||1.96 |
| + | |- | ||
| + | |9/2 14:00 Spinach||438.3||1.86||1.99 | ||
| + | |- | ||
| + | |9/2 14:00 tetR-apt 12_1R||567.5||1.90||1.99 | ||
| + | |- | ||
| + | |9/2 21:00 pT181 attenuator||320.9||1.90||2.15 | ||
| + | |- | ||
| + | |9/2 21:00 Spinach||457.0||1.88||2.24 | ||
|} | |} | ||
</div> | </div> | ||
| + | |||
===Liquid Culture=== | ===Liquid Culture=== | ||
| + | <!-- ここから --> | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Kojima</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||medium | ||
| + | |- | ||
| + | |Spinach-DT(9/2 master plate)||plusgrow(CP)4ml | ||
| + | |} | ||
| + | </div> | ||
| + | <!-- ここまでをコピーしてね --> | ||
| + | |||
===Restriction Enzyme Digestion=== | ===Restriction Enzyme Digestion=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Kojima</span> | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||EcoRI||SpeI||XbaI||PstI||10xBuffer B(EcoRI&SpeI)||10xBuffer D(XbaI&PstI)||100xBSA||MilliQ||total | ||
| + | |- | ||
| + | |9/3 pT181attenuator 14:00 418.4ng/µL|| 4.8µL|| 1.0µL|| 1.0µL|| 0µL|| 0µL|| 3µL|| 0µL|| 0.3µL|| 19.9µL|| 30µL | ||
| + | |- | ||
| + | |9/3 pT181attenuator 14:00 418.4ng/µL|| 0.2µL|| 0µL|| 0µL|| 0µL|| 0µL|| 1µL|| 0µL|| 0.1µL|| 8.7µL|| 10µL | ||
| + | |- | ||
| + | |9/3 pT181attenuator 14:00 418.4ng/µL|| 4.8µL|| 0µL|| 0µL|| 1.0µL|| 1.0µL|| 0µL|| 3µL|| 0.3µL|| 19.9µL|| 30µL | ||
| + | |- | ||
| + | |9/3 pT181attenuator 14:00 418.4ng/µL|| 0.2µL|| 0µL|| 0µL|| 0µL|| 0µL|| 0µL|| 1µL|| 0.1µL|| 8.7µL|| 10µL | ||
| + | |} | ||
| + | </div> | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||EcoRI||SpeI||XbaI||PstI||10xBuffer B(EcoRI&SpeI)||10xBuffer D(XbaI&PstI)||100xBSA||MilliQ||total | ||
| + | |- | ||
| + | |9/3 pT181antisense 14:00 362.2ng/µL|| 5.5µL|| 1.0µL|| 1.0µL|| 0µL|| 0µL|| 3µL|| 0µL|| 0.3µL|| 19.2µL|| 30µL | ||
| + | |- | ||
| + | |9/3 pT181attenuator 14:00 418.4ng/µL|| 0.3µL|| 0µL|| 0µL|| 0µL|| 0µL|| 1µL|| 0µL|| 0.1µL|| 8.6µL|| 10µL | ||
| + | |- | ||
| + | |9/3 pT181attenuator 14:00 418.4ng/µL|| 5.5µL|| 0µL|| 0µL|| 1.0µL|| 1.0µL|| 0µL|| 3µL|| 0.3µL|| 19.2µL|| 30µL | ||
| + | |- | ||
| + | |9/3 pT181attenuator 14:00 418.4ng/µL|| 0.3µL|| 0µL|| 0µL|| 0µL|| 0µL|| 0µL|| 1µL|| 0.1µL|| 8.6µL|| 10µL | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||EcoRI||SpeI||10xBuffer B||100xBSA||MilliQ||total | ||
| + | |- | ||
| + | |9/3 apt 12_1R 14:00 507.5ng/µL|| 3.9µL|| 1.0µL|| 1.0µL|| 3µL|| 0.3µL|| 20.8µL|| 30µL | ||
| + | |- | ||
| + | |9/3 apt 12_1R 14:00 507.5ng/µL|| 0.2µL|| 0µL|| 0µL|| 1µL|| 0.1µL|| 8.7µL|| 10µL | ||
| + | |} | ||
| + | </div> | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||EcoRI||XbaI||10xBuffer D||100xBSA||MilliQ||total | ||
| + | |- | ||
| + | |8/29 DT 166.2ng/µL|| 12µL|| 1.0µL|| 1.0µL|| 3µL|| 0.3µL|| 12.7µL|| 30µL | ||
| + | |- | ||
| + | |8/29 DT 166.2ng/µL|| 0.6µL|| 0µL|| 0µL|| 1µL|| 0.1µL|| 8.3µL|| 10µL | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||EcoRI||SpeI||10xBuffer B||100xBSA||MilliQ||total | ||
| + | |- | ||
| + | |8/22 RBS-lysis1(2) 96ng/µL|| 21µL|| 1.0µL|| 1.0µL|| 3µL|| 0.3µL|| 3.7µL|| 30µL | ||
| + | |- | ||
| + | |8/22 RBS-lysis1(2) 96ng/µL|| 1.0µL|| 0µL|| 0µL|| 1µL|| 0.1µL|| 7.9µL|| 10µL | ||
| + | |} | ||
| + | </div> | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||EcoRI||SpeI||10xBuffer B||100xBSA||MilliQ||total | ||
| + | |- | ||
| + | |8/30 RBS-lysis2 220ng/µL|| 9.1µL|| 1.0µL|| 1.0µL|| 3µL|| 0.3µL|| 15.6µL|| 30µL | ||
| + | |- | ||
| + | |8/30 RBS-lysis2 220ng/µL|| 0.5µL|| 0µL|| 0µL|| 1µL|| 0.1µL|| 8.4µL|| 10µL | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | ! ||DNA||EcoRI||SpeI||10xBuffer B||100xBSA||MilliQ||total | ||
| + | |- | ||
| + | |8/20 RBS-lysis3(1) 282ng/µL|| 7.1µL|| 1.0µL|| 1.0µL|| 3µL|| 0.3µL|| 17.6µL|| 30µL | ||
| + | |- | ||
| + | |8/20 RBS-lysis3(1) 282ng/µL|| 0.4µL|| 0µL|| 0µL|| 1µL|| 0.1µL|| 8.5µL|| 10µL | ||
| + | |} | ||
| + | </div> | ||
| + | |||
===PCR=== | ===PCR=== | ||
| + | <!-- ここから --> | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Hirano</span> | ||
| + | {| class="wikitable" | ||
| + | !Plac(1) plasmid 153.7ng/µL||10x buffer KOD plus-ver.2|||2mM dNTPs||25mM MgSO4||primer (KaiA_BsaI_Mut_fwd)||primer (KaiA_BsaI_Mut_rer)||KOD plus-ver.2||MilliQ||total | ||
| + | |- | ||
| + | |0.13 ||2.5||2.5||1.5||0.75||0.75||0.5||16.37||25 | ||
| + | |} | ||
| + | </div> | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | !KaiB (plasmid) 20.2ng/µL||10x buffer KOD plus-ver.2|||2mM dNTPs||25mM MgSO4||primer (GG2_RBS_KaiB_fwd)||primer (KaiB_GG3_rer)||KOD plus-ver.2||MilliQ||total | ||
| + | |- | ||
| + | |1||2.5||2.5||1.5||0.75||0.75||0.5||15.5||25 | ||
| + | |} | ||
| + | </div> | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | !KaiC (plasmid) 4.4ng/µL||10x buffer KOD plus-ver.2|||2mM dNTPs||25mM MgSO4||primer ||primer ||KOD plus-ver.2||MilliQ||total | ||
| + | |- | ||
| + | |1||2.5||2.5||1.5||0.75||0.75||0.5||15.5||25 | ||
| + | |} | ||
| + | </div> | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | !DT-1 (plasmid) 153.7ng/µL||10x buffer KOD plus-ver.2|||2mM dNTPs||25mM MgSO4||primer ||primer ||KOD plus-ver.2||MilliQ||total | ||
| + | |- | ||
| + | |0.1||2.5||2.5||1.5||0.75||0.75||0.5||16.4||25 | ||
| + | |} | ||
| + | </div> | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | !PKaiBC(PCR product) 16.3ng/µL||10x buffer KOD plus-ver.2|||2mM dNTPs||25mM MgSO4||primer ||primer ||KOD plus-ver.2||MilliQ||total | ||
| + | |- | ||
| + | |0.7||2.5||2.5||1.5||0.75||0.75||0.5||15.8||25 | ||
| + | |} | ||
| + | </div> | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | !SasA(PCR product) 21.6ng/µL||10x buffer KOD plus-ver.2|||2mM dNTPs||25mM MgSO4||primer ||primer ||KOD plus-ver.2||MilliQ||total | ||
| + | |- | ||
| + | |0.5||2.5||2.5||1.5||0.75||0.75||0.5||16||25 | ||
| + | |} | ||
| + | </div> | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | !RpaA(PCR product) 15.4ng/µL||10x buffer KOD plus-ver.2|||2mM dNTPs||25mM MgSO4||primer ||primer ||KOD plus-ver.2||MilliQ||total | ||
| + | |- | ||
| + | |0.6||2.5||2.5||1.5||0.75||0.75||0.5||15.9||25 | ||
| + | |} | ||
| + | </div> | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | !RpaB(PCR product) 26.7ng/µL||10x buffer KOD plus-ver.2|||2mM dNTPs||25mM MgSO4||primer ||primer ||KOD plus-ver.2||MilliQ||total | ||
| + | |- | ||
| + | |0.4||2.5||2.5||1.5||0.75||0.75||0.5||16.1||25 | ||
| + | |} | ||
| + | </div> | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94°C||98°C||||68°C||-- | ||
| + | |- | ||
| + | |5min||10sec||30sec||||30 | ||
| + | |} | ||
| + | <div class="experiment"> | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94°C||98°C||||68°C||-- | ||
| + | |- | ||
| + | |5min||10sec||30sec||||30 | ||
| + | |} | ||
| + | |||
| + | |||
| + | <!-- ここまでをコピーしてね --> | ||
| + | |||
===Mutatation PCR=== | ===Mutatation PCR=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !KaiABC(12.6pg/µL)||Primer(Kai_BsaIMut_fwd)||Primer(Kai_BsaIMut_rer)||Prime STAR Max Premix||MilliQ||total | ||
| + | |- | ||
| + | |1 ||1||1||25||22||50 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !||Primer(Kai_BsaIMut_fwd)||Primer(Kai_BsaIMut_rer)||Prime STAR Max Premix||MilliQ||total | ||
| + | |- | ||
| + | | ||1||1||25||23||50 | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | | 94°C|| 98°C|| 65°C|| 72°C||-- | ||
| + | |- | ||
| + | |5min||10sec ||15sec ||40sec ||30 | ||
| + | |} | ||
| + | </div> | ||
| + | |||
===Colony PCR=== | ===Colony PCR=== | ||
| - | === | + | <!-- ここから --> |
| + | <div class="experiment"> | ||
| + | <span class="author"></span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||base pair | ||
| + | |- | ||
| + | |9/1 pT181antisense(pSB1C3)||400bp | ||
| + | |- | ||
| + | |9/1 pT181antisense-DT||547bp | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | | 94°C|| 94°C|| 55°C|| 68°C||-- | ||
| + | |- | ||
| + | |5m ||30s ||30s ||30s ||30 | ||
| + | |} | ||
| + | </div> | ||
| + | ===Electrophoresis=== | ||
| + | |||
| + | <div class="experiment"> | ||
| + | <span class="author">Kojima</span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1||100bp ladder | ||
| + | |- | ||
| + | |2||9/1 pT181antisense(pSB1C3) | ||
| + | |- | ||
| + | |3||9/1 pT181antisense | ||
| + | |} | ||
| + | [[File:Igku Sep3 Electrophoresis (N2).jpg]]<br> | ||
| + | </div> | ||
| + | |||
| + | ===Electrophoresis=== | ||
| + | |||
| + | <!-- こっから --> | ||
| + | <div class="experiment"> | ||
| + | <span class="author"></span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1 ||100bp ladder | ||
| + | |- | ||
| + | |2 ||attenuator EcoRI&SpeI | ||
| + | |- | ||
| + | |3 ||attenuator negative EcoRI&SpeI | ||
| + | |- | ||
| + | |4 ||attenuator XbaI&PstI | ||
| + | |- | ||
| + | |5 ||attenuator negative XbaI&PstI | ||
| + | |- | ||
| + | |6 ||antisense EcoRI&SpeI | ||
| + | |- | ||
| + | |7 ||antisense negative EcoRI&SpeI | ||
| + | |- | ||
| + | |8 ||100bp ladder | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1 ||100bp ladder | ||
| + | |- | ||
| + | |2 ||antisense XbaI&PstI | ||
| + | |- | ||
| + | |3 ||antisense negative XbaI&PstI | ||
| + | |- | ||
| + | |4 ||apt EcoRI&SpeI | ||
| + | |- | ||
| + | |5 ||apt negative EcoRI&SpeI | ||
| + | |- | ||
| + | |6 ||DT EcoRI&XbaI | ||
| + | |- | ||
| + | |7 ||DT negative EcoRI&XbaI | ||
| + | |- | ||
| + | |8 ||100bp ladder | ||
| + | |} | ||
| + | [[File:Igku Sep3 Electrophoresis(N3_2).jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Lane||Sample | ||
| + | |- | ||
| + | |1 ||100bp ladder | ||
| + | |- | ||
| + | |2 ||RBS-lysis1 EcoRI&SpeI | ||
| + | |- | ||
| + | |3 ||RBS-lysis1 negative | ||
| + | |- | ||
| + | |4 ||RBS-lysis2 EcoRI&SpeI | ||
| + | |- | ||
| + | |5 ||RBS-lysis2 negative | ||
| + | |- | ||
| + | |6 ||RBS-lysis3 EcoRI&SpeI | ||
| + | |- | ||
| + | |7 ||RBS-lysis3 negative | ||
| + | |- | ||
| + | |8 ||100bp ladder | ||
| + | |} | ||
| + | [[File:Igku Sep3 Electrophoresis(N3_3).jpg]]<br> | ||
| + | </div> | ||
| + | |||
===Gel Extraction=== | ===Gel Extraction=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author"></span> | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1 ||100bp ladder || | ||
| + | |- | ||
| + | |3 ||pT181 attenuator||EcoRI&SpeI | ||
| + | |- | ||
| + | |4 ||pT181 attenuator||EcoRI&SpeI | ||
| + | |- | ||
| + | |6 ||pT181 attenuator||XbaI&PstI | ||
| + | |- | ||
| + | |7 ||pT181 attenuator||XbaI&PstI | ||
| + | |- | ||
| + | |9 ||pT181 antisense||EcoRI&SpeI | ||
| + | |- | ||
| + | |10 ||pT181 antisense||EcoRI&SpeI | ||
| + | |} | ||
| + | [[File:Igku Sep3 Electrophoresis(N4_1).jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1 ||100bp ladder || | ||
| + | |- | ||
| + | |2 ||pT181 antisense||XbaI&PstI | ||
| + | |- | ||
| + | |3 ||pT181 antisense||XbaI&PstI | ||
| + | |- | ||
| + | |5 ||apt12_1R||EcoRI&SpeI | ||
| + | |- | ||
| + | |6 ||apt12_1R||EcoRI&SpeI | ||
| + | |} | ||
| + | [[File:Igku Sep3 Electrophoresis(N4_2).jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1 ||100bp ladder || | ||
| + | |- | ||
| + | |2 ||DT||EcoRI&XbaI | ||
| + | |- | ||
| + | |3 ||DT||EcoRI&XbaI | ||
| + | |- | ||
| + | |5 ||RBS-lysis1||EcoRI&SpeI | ||
| + | |- | ||
| + | |6 ||RBS-lysis1||EcoRI&SpeI | ||
| + | |} | ||
| + | [[File:Igku Sep3 Electrophoresis(N5_3).jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Lane||DNA||Enzyme | ||
| + | |- | ||
| + | |1 ||100bp ladder || | ||
| + | |- | ||
| + | |2 ||RBS-lysis2||EcoRI&SpeI | ||
| + | |- | ||
| + | |3 ||RBS-lysis2||EcoRI&SpeI | ||
| + | |- | ||
| + | |5 ||RBS-lysis3||EcoRI&SpeI | ||
| + | |- | ||
| + | |6 ||RBS-lysis3||EcoRI&SpeI | ||
| + | |} | ||
| + | [[File:Igku Sep3 Electrophoresis(N5_4).jpg]]<br> | ||
| + | {| class="wikitable" | ||
| + | !Name||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |pT181 attenuator(EcoRI&SpeI)||10.0 ||1.86 ||0.39 | ||
| + | |- | ||
| + | |pT181 attenuator(XbaI&PstI) ||11.7 ||1.65 ||0.36 | ||
| + | |- | ||
| + | |pT181 antisense(EcoRI&SpeI) ||4.2 ||1.95 ||0.25 | ||
| + | |- | ||
| + | |pT181 antisense(XbaI&PstI)||6.6 ||1.78 ||0.33 | ||
| + | |- | ||
| + | |tRNA 12_1R apt(EcoRI&SpeI)||5.7 ||1.73 ||0.29 | ||
| + | |- | ||
| + | |DT(EcoRI&XbaI) ||16.6 ||1.99 ||0.76 | ||
| + | |- | ||
| + | |RBS-lysis1(EcoRI&SpeI)||8.8 ||1.65 ||0.32 | ||
| + | |- | ||
| + | |RBS-lysis2(EcoRI&SpeI)||13.4 ||1.61 ||0.48 | ||
| + | |- | ||
| + | |RBS-lysis3(EcoRI&SpeI)||10.2 ||2.19 ||0.51 | ||
| + | |} | ||
| + | </div> | ||
| + | |||
===Liquid Culture=== | ===Liquid Culture=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||medium | ||
| + | |- | ||
| + | |8/16master plate7(DT) || | ||
| + | |- | ||
| + | |8/21RBS-lysis1 || | ||
| + | |} | ||
| + | </div> | ||
Latest revision as of 03:54, 28 September 2013
Contents |
Sep 3
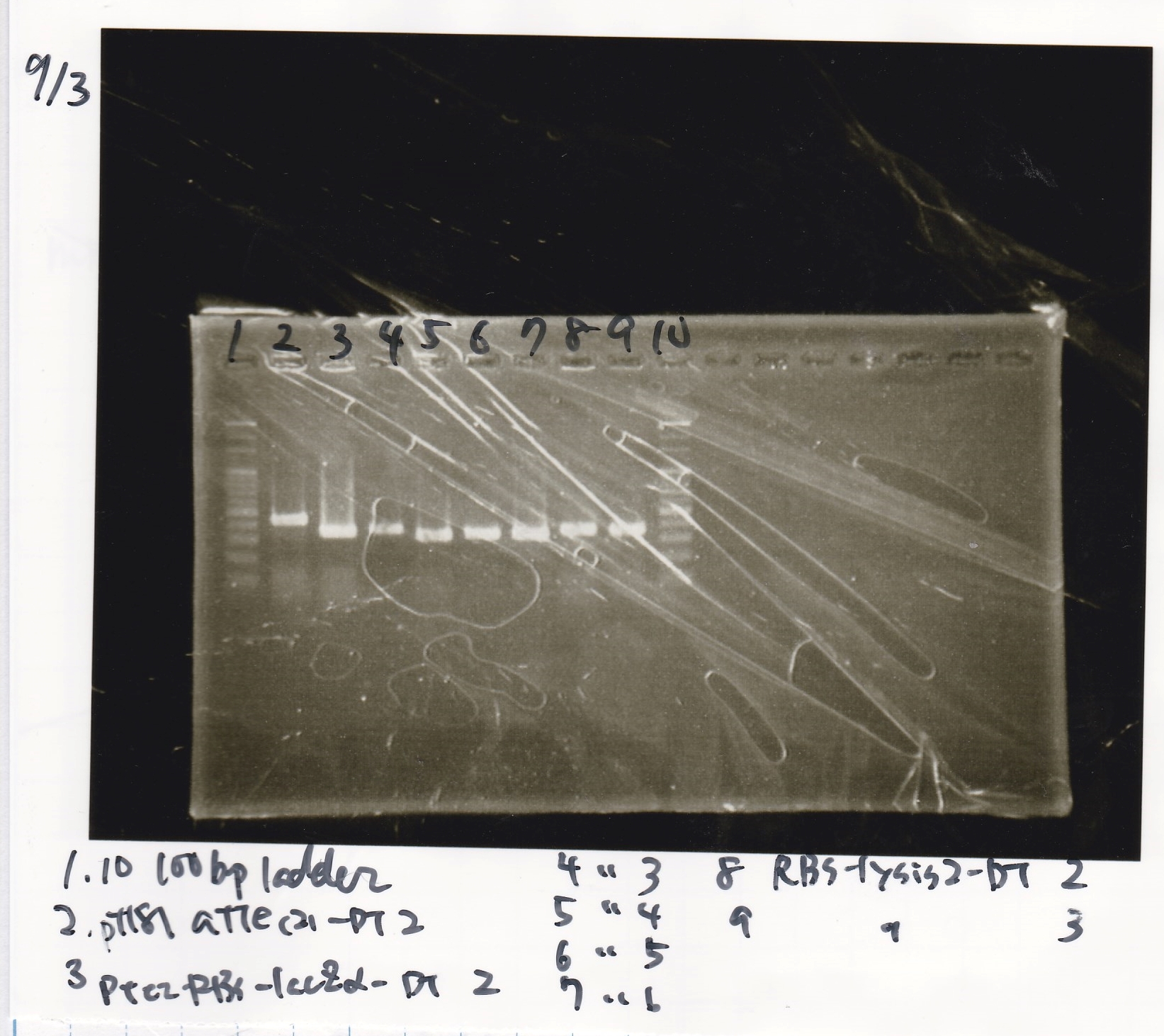
Electrophoresis
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | pT181 attenuator(2)-DT2(738) |
| 3 | Ptet-RBS-lacZα-DT-2(756) |
| 4 | Ptet-RBS-lacZα-DT-3 |
| 5 | Ptet-RBS-lacZα-DT-4 |
| 6 | Ptet-RBS-lacZα-DT-5 |
| 7 | Ptet-RBS-lacZα-DT-6 |
| 8 | RBS-lysis2-DT-2(985) |
| 9 | RBS-lysis2-DT-3 |
| 10 | 100bp ladder |
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 9/2 14:00 pT181 attenuator | 418.4 | 1.82 | 1.80 |
| 9/2 14:00 pT181 antisense | 362.2 | 1.92 | 1.96 |
| 9/2 14:00 Spinach | 438.3 | 1.86 | 1.99 |
| 9/2 14:00 tetR-apt 12_1R | 567.5 | 1.90 | 1.99 |
| 9/2 21:00 pT181 attenuator | 320.9 | 1.90 | 2.15 |
| 9/2 21:00 Spinach | 457.0 | 1.88 | 2.24 |
Liquid Culture
| Sample | medium |
|---|---|
| Spinach-DT(9/2 master plate) | plusgrow(CP)4ml |
Restriction Enzyme Digestion
| DNA | EcoRI | SpeI | XbaI | PstI | 10xBuffer B(EcoRI&SpeI) | 10xBuffer D(XbaI&PstI) | 100xBSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|---|
| 9/3 pT181attenuator 14:00 418.4ng/µL | 4.8µL | 1.0µL | 1.0µL | 0µL | 0µL | 3µL | 0µL | 0.3µL | 19.9µL | 30µL |
| 9/3 pT181attenuator 14:00 418.4ng/µL | 0.2µL | 0µL | 0µL | 0µL | 0µL | 1µL | 0µL | 0.1µL | 8.7µL | 10µL |
| 9/3 pT181attenuator 14:00 418.4ng/µL | 4.8µL | 0µL | 0µL | 1.0µL | 1.0µL | 0µL | 3µL | 0.3µL | 19.9µL | 30µL |
| 9/3 pT181attenuator 14:00 418.4ng/µL | 0.2µL | 0µL | 0µL | 0µL | 0µL | 0µL | 1µL | 0.1µL | 8.7µL | 10µL |
| DNA | EcoRI | SpeI | XbaI | PstI | 10xBuffer B(EcoRI&SpeI) | 10xBuffer D(XbaI&PstI) | 100xBSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|---|
| 9/3 pT181antisense 14:00 362.2ng/µL | 5.5µL | 1.0µL | 1.0µL | 0µL | 0µL | 3µL | 0µL | 0.3µL | 19.2µL | 30µL |
| 9/3 pT181attenuator 14:00 418.4ng/µL | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 0µL | 0.1µL | 8.6µL | 10µL |
| 9/3 pT181attenuator 14:00 418.4ng/µL | 5.5µL | 0µL | 0µL | 1.0µL | 1.0µL | 0µL | 3µL | 0.3µL | 19.2µL | 30µL |
| 9/3 pT181attenuator 14:00 418.4ng/µL | 0.3µL | 0µL | 0µL | 0µL | 0µL | 0µL | 1µL | 0.1µL | 8.6µL | 10µL |
| DNA | EcoRI | SpeI | 10xBuffer B | 100xBSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 9/3 apt 12_1R 14:00 507.5ng/µL | 3.9µL | 1.0µL | 1.0µL | 3µL | 0.3µL | 20.8µL | 30µL |
| 9/3 apt 12_1R 14:00 507.5ng/µL | 0.2µL | 0µL | 0µL | 1µL | 0.1µL | 8.7µL | 10µL |
| DNA | EcoRI | XbaI | 10xBuffer D | 100xBSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 8/29 DT 166.2ng/µL | 12µL | 1.0µL | 1.0µL | 3µL | 0.3µL | 12.7µL | 30µL |
| 8/29 DT 166.2ng/µL | 0.6µL | 0µL | 0µL | 1µL | 0.1µL | 8.3µL | 10µL |
| DNA | EcoRI | SpeI | 10xBuffer B | 100xBSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 8/22 RBS-lysis1(2) 96ng/µL | 21µL | 1.0µL | 1.0µL | 3µL | 0.3µL | 3.7µL | 30µL |
| 8/22 RBS-lysis1(2) 96ng/µL | 1.0µL | 0µL | 0µL | 1µL | 0.1µL | 7.9µL | 10µL |
| DNA | EcoRI | SpeI | 10xBuffer B | 100xBSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 8/30 RBS-lysis2 220ng/µL | 9.1µL | 1.0µL | 1.0µL | 3µL | 0.3µL | 15.6µL | 30µL |
| 8/30 RBS-lysis2 220ng/µL | 0.5µL | 0µL | 0µL | 1µL | 0.1µL | 8.4µL | 10µL |
| DNA | EcoRI | SpeI | 10xBuffer B | 100xBSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 8/20 RBS-lysis3(1) 282ng/µL | 7.1µL | 1.0µL | 1.0µL | 3µL | 0.3µL | 17.6µL | 30µL |
| 8/20 RBS-lysis3(1) 282ng/µL | 0.4µL | 0µL | 0µL | 1µL | 0.1µL | 8.5µL | 10µL |
PCR
| Plac(1) plasmid 153.7ng/µL | 10x buffer KOD plus-ver.2 | 2mM dNTPs | 25mM MgSO4 | primer (KaiA_BsaI_Mut_fwd) | primer (KaiA_BsaI_Mut_rer) | KOD plus-ver.2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.13 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 16.37 | 25 |
| KaiB (plasmid) 20.2ng/µL | 10x buffer KOD plus-ver.2 | 2mM dNTPs | 25mM MgSO4 | primer (GG2_RBS_KaiB_fwd) | primer (KaiB_GG3_rer) | KOD plus-ver.2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 15.5 | 25 |
| KaiC (plasmid) 4.4ng/µL | 10x buffer KOD plus-ver.2 | 2mM dNTPs | 25mM MgSO4 | primer | primer | KOD plus-ver.2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 15.5 | 25 |
| DT-1 (plasmid) 153.7ng/µL | 10x buffer KOD plus-ver.2 | 2mM dNTPs | 25mM MgSO4 | primer | primer | KOD plus-ver.2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.1 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 16.4 | 25 |
| PKaiBC(PCR product) 16.3ng/µL | 10x buffer KOD plus-ver.2 | 2mM dNTPs | 25mM MgSO4 | primer | primer | KOD plus-ver.2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.7 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 15.8 | 25 |
| SasA(PCR product) 21.6ng/µL | 10x buffer KOD plus-ver.2 | 2mM dNTPs | 25mM MgSO4 | primer | primer | KOD plus-ver.2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 16 | 25 |
| RpaA(PCR product) 15.4ng/µL | 10x buffer KOD plus-ver.2 | 2mM dNTPs | 25mM MgSO4 | primer | primer | KOD plus-ver.2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.6 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 15.9 | 25 |
| RpaB(PCR product) 26.7ng/µL | 10x buffer KOD plus-ver.2 | 2mM dNTPs | 25mM MgSO4 | primer | primer | KOD plus-ver.2 | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.4 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 16.1 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 68°C | -- | |
| 5min | 10sec | 30sec | 30 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 68°C | -- | |
| 5min | 10sec | 30sec | 30 |
Mutatation PCR
| KaiABC(12.6pg/µL) | Primer(Kai_BsaIMut_fwd) | Primer(Kai_BsaIMut_rer) | Prime STAR Max Premix | MilliQ | total |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 25 | 22 | 50 |
| Primer(Kai_BsaIMut_fwd) | Primer(Kai_BsaIMut_rer) | Prime STAR Max Premix | MilliQ | total | |
|---|---|---|---|---|---|
| 1 | 1 | 25 | 23 | 50 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 65°C | 72°C | -- |
| 5min | 10sec | 15sec | 40sec | 30 |
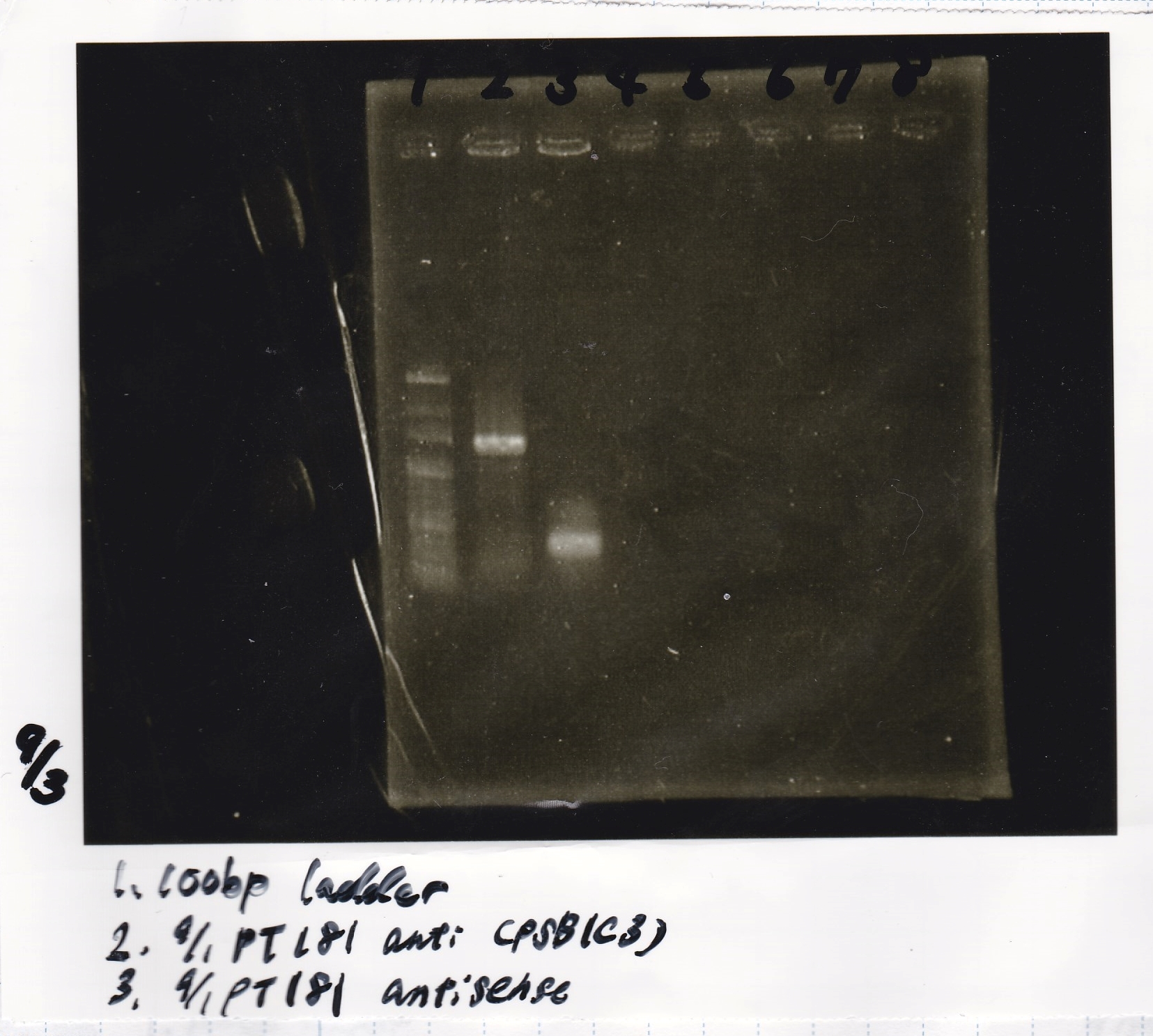
Colony PCR
| Sample | base pair |
|---|---|
| 9/1 pT181antisense(pSB1C3) | 400bp |
| 9/1 pT181antisense-DT | 547bp |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5m | 30s | 30s | 30s | 30 |
Electrophoresis
Electrophoresis
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | attenuator EcoRI&SpeI |
| 3 | attenuator negative EcoRI&SpeI |
| 4 | attenuator XbaI&PstI |
| 5 | attenuator negative XbaI&PstI |
| 6 | antisense EcoRI&SpeI |
| 7 | antisense negative EcoRI&SpeI |
| 8 | 100bp ladder |
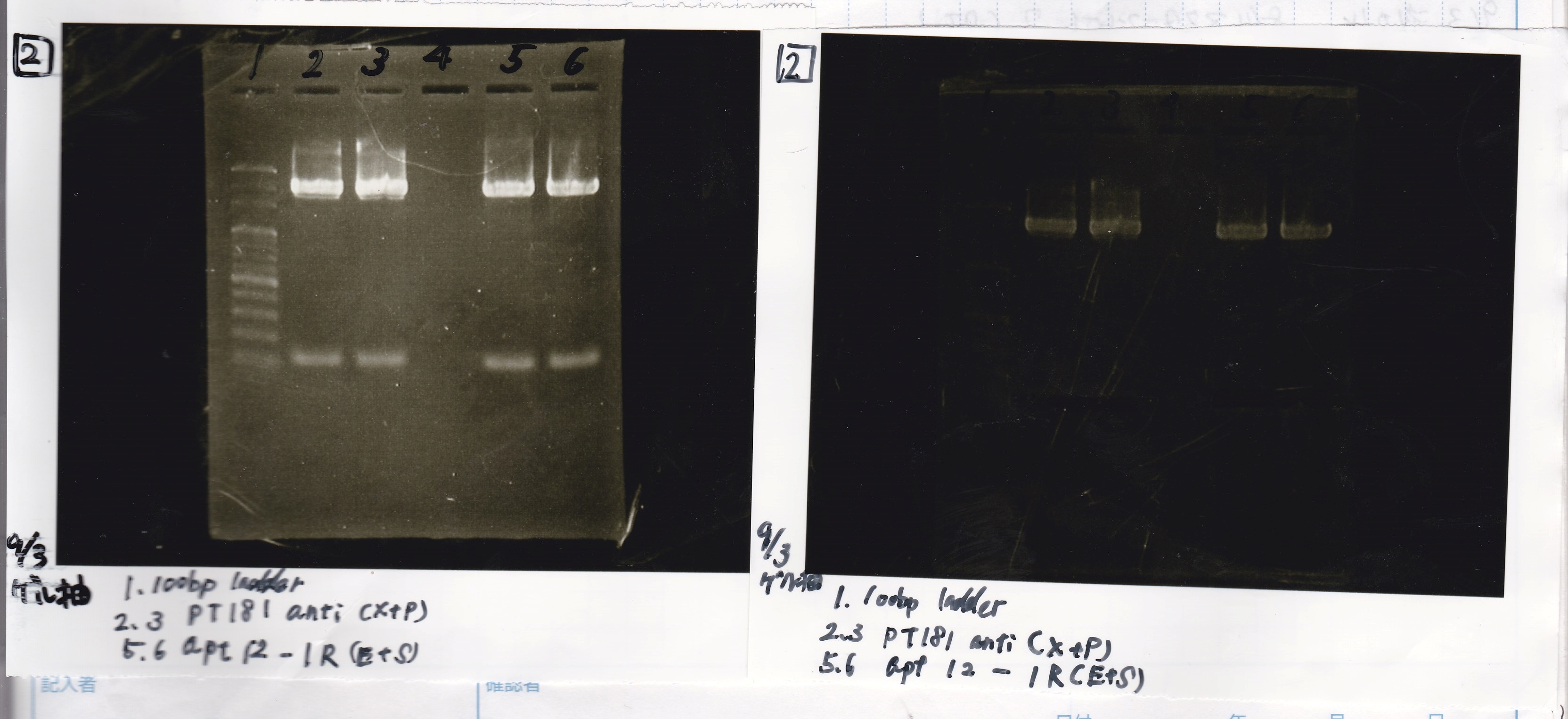
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | antisense XbaI&PstI |
| 3 | antisense negative XbaI&PstI |
| 4 | apt EcoRI&SpeI |
| 5 | apt negative EcoRI&SpeI |
| 6 | DT EcoRI&XbaI |
| 7 | DT negative EcoRI&XbaI |
| 8 | 100bp ladder |
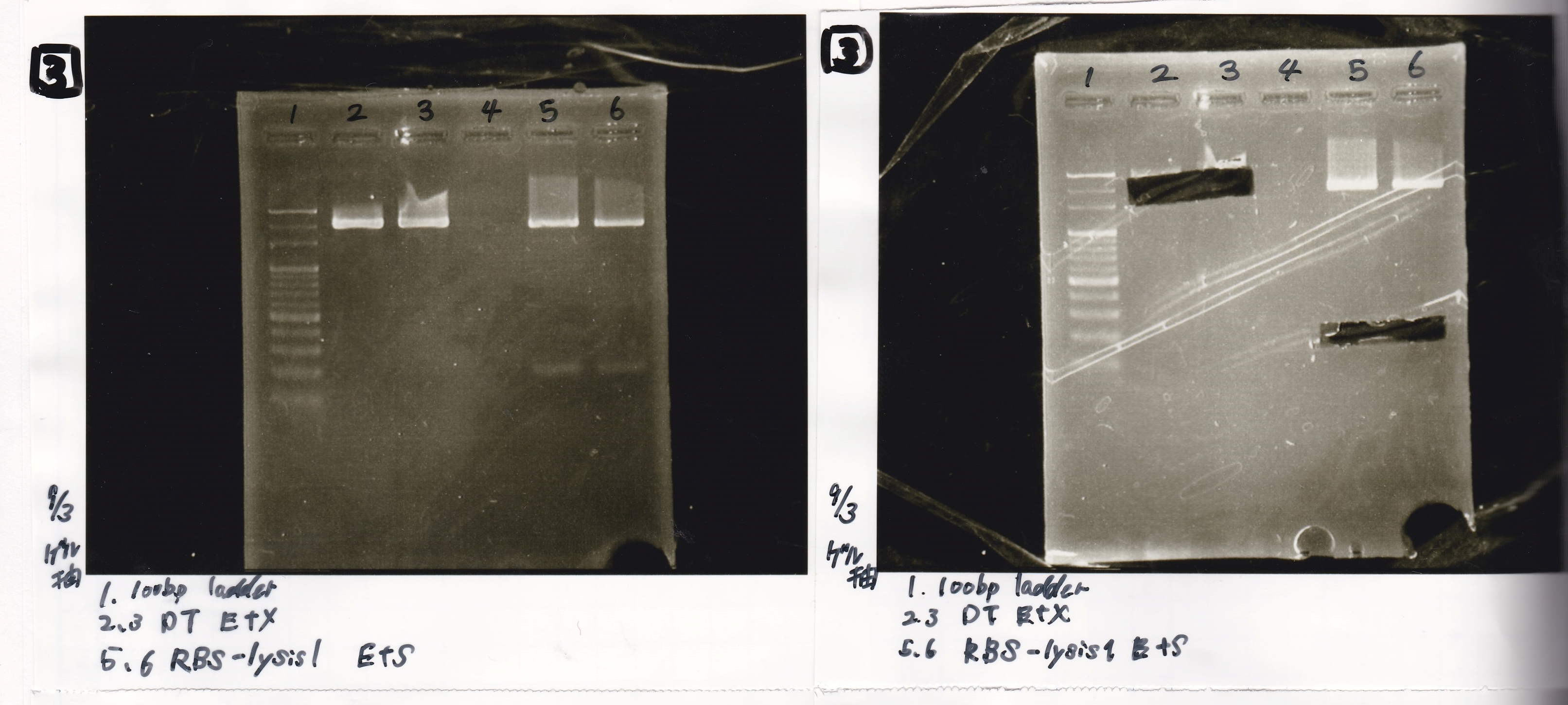
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | RBS-lysis1 EcoRI&SpeI |
| 3 | RBS-lysis1 negative |
| 4 | RBS-lysis2 EcoRI&SpeI |
| 5 | RBS-lysis2 negative |
| 6 | RBS-lysis3 EcoRI&SpeI |
| 7 | RBS-lysis3 negative |
| 8 | 100bp ladder |
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | |
| 3 | pT181 attenuator | EcoRI&SpeI |
| 4 | pT181 attenuator | EcoRI&SpeI |
| 6 | pT181 attenuator | XbaI&PstI |
| 7 | pT181 attenuator | XbaI&PstI |
| 9 | pT181 antisense | EcoRI&SpeI |
| 10 | pT181 antisense | EcoRI&SpeI |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | |
| 2 | pT181 antisense | XbaI&PstI |
| 3 | pT181 antisense | XbaI&PstI |
| 5 | apt12_1R | EcoRI&SpeI |
| 6 | apt12_1R | EcoRI&SpeI |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | |
| 2 | DT | EcoRI&XbaI |
| 3 | DT | EcoRI&XbaI |
| 5 | RBS-lysis1 | EcoRI&SpeI |
| 6 | RBS-lysis1 | EcoRI&SpeI |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | |
| 2 | RBS-lysis2 | EcoRI&SpeI |
| 3 | RBS-lysis2 | EcoRI&SpeI |
| 5 | RBS-lysis3 | EcoRI&SpeI |
| 6 | RBS-lysis3 | EcoRI&SpeI |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| pT181 attenuator(EcoRI&SpeI) | 10.0 | 1.86 | 0.39 |
| pT181 attenuator(XbaI&PstI) | 11.7 | 1.65 | 0.36 |
| pT181 antisense(EcoRI&SpeI) | 4.2 | 1.95 | 0.25 |
| pT181 antisense(XbaI&PstI) | 6.6 | 1.78 | 0.33 |
| tRNA 12_1R apt(EcoRI&SpeI) | 5.7 | 1.73 | 0.29 |
| DT(EcoRI&XbaI) | 16.6 | 1.99 | 0.76 |
| RBS-lysis1(EcoRI&SpeI) | 8.8 | 1.65 | 0.32 |
| RBS-lysis2(EcoRI&SpeI) | 13.4 | 1.61 | 0.48 |
| RBS-lysis3(EcoRI&SpeI) | 10.2 | 2.19 | 0.51 |
Liquid Culture
| Sample | medium |
|---|---|
| 8/16master plate7(DT) | |
| 8/21RBS-lysis1 |
 "
"